

A Mononuclear Iron Thiolate Complex with N-Heterocyclic Carbene Ligation
Received date: 2022-01-21
Online published: 2022-02-15
Supported by
National Natural Science Foundation of China(21725104); National Natural Science Foundation of China(22061160464); National Natural Science Foundation of China(21821002); Program of Shanghai Academic Research Leader(19XD1424800); K. C. Wong Education Foundation
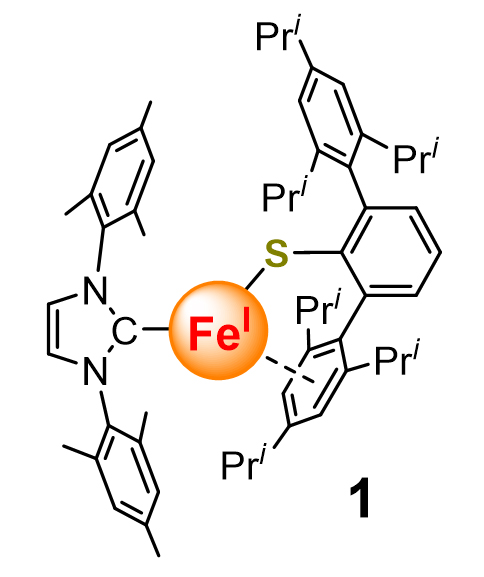
The relevance of low-valent iron complexes bearing sulfur-containing ligands with the reactive iron intermediates in enzymatic dinitrogen fixation has stimulated great synthetic efforts toward them. Low-valent iron thiolate complexes have been mostly known for the thiolate-bridged diiron(I) carbonyl compounds. In contrast, isolable mononuclear iron(I) thiolate complexes are exceedingly rare. Herein, we report the synthesis and characterization of a mononuclear iron(I) complex [(IMes)Fe(SAr*)] (1, IMes=1,3-bis(mesityl)imidazole-2-ylidene, Ar*=2,6-(2',4',6'-Pri3C6H2)2C6H3) as well as its catalytic performance in dinitrogen silylation reaction. Complex 1 was synthesized from the reaction of (IMes)2FeBr with KSAr* in Et2O in 81% isolated yield. Single-crystal X-ray diffraction studies revealed that the thiolate ligand in 1 is coordinating to the iron center in an σ:η6-fashion via the sulfur atom and one of the flanking Pri3C6H2 ring with the Fe—S distance of 0.2228(1) nm and the Fe—C(Trip) distances ranging from 0.2076(2) to 0.2205(2) nm. Zero-field 57Fe Mössbauer spectrum at 80 K (δ=0.56 mm•s-1; ΔEQ=0.98 mm•s-1) and X-band electron paramagnetic resonance data (g=[2.20, 2.03, 1.99]) at 103 K of 1 point to its low-spin iron(I) nature (S=1/2). These data are comparable with those of Holland's iron(I) thiolate complex K[(2-S-1,3-(6-F-3-(2,4,6-Pri3C6H2)C6H2)2C6H4)Fe] (Nature 2015, 526, 96). Density functional theory studies support the S=1/2 ground spin-state for 1 and reveal that the interaction between the iron atom and the sulfur atom is essentially σ-bonding in character. In addition, π- and δ-type interactions between the iron atom and the η6-Pri3C6H2 ring in 1 also exist. Complex 1 can catalyze the reaction of N2 with KC8 and Me3SiCl in Et2O at room temperature, affording N(SiMe3)3 with a turn-over number up to 73 in 24 h and 87 in 48 h. The reaction employing (IMes)2FeBr as catalyst has a turn-over number up to 73 in 24 h and elongating the reaction time to 48 h does not lead to further increase of the turn-over number. The fine catalytic performance with 1 over (IMes)2FeBr implies that the thiolate ligand in 1 might render the catalytically active intermediate a relatively long lifetime.
Key words: N-heterocyclic carbene; iron; sulfur ligand; N2 activation; low valent
Shangzhao Li , Zhenwu Ouyang , Junjie Zou , Dongyang Wang , Bin Xu , Liang Deng . A Mononuclear Iron Thiolate Complex with N-Heterocyclic Carbene Ligation[J]. Acta Chimica Sinica, 2022 , 80(3) : 272 -276 . DOI: 10.6023/A22010038
| [1] | (a) Zhang, S.; Lu, H.; Kang, Q.; Huang, Y.; Fu, X. Sci. Sin. Chim. 2021, 51, 538. |
| [1] | (b) Zhang, Y.; Ma, X.; Zhang, X.; Lei, M. Acta Chim. Sinica 2016, 74, 340. (in Chinese) |
| [1] | (张益伟, 马雪璐, 张欣, 雷鸣, 化学学报, 2016, 74, 340.) |
| [1] | (c) Yue, G.; Gao, R.; Zhao, P.; Chu, M.; Shuai, M. Acta Chim. Sinica 2016, 74, 657. (in Chinese) |
| [1] | (岳国宗, 高瑞, 赵鹏翔, 褚明福, 帅茂兵, 化学学报, 2016, 74, 657.) |
| [1] | (d) Li, J.; Yin, J.; Yu, C.; Zhang, W.; Xi, Z. Acta Chim. Sinica 2017, 75, 733. (in Chinese) |
| [1] | (李嘉鹏, 殷剑昊, 俞超, 张文雄, 席振峰, 化学学报, 2017, 75, 733.) |
| [1] | (e) Lv, Z.; Wei, J.; Zhang, W.; Chen, P.; Deng, D.; Shi, Z.; Xi, Z. Natl. Sci. Rev. 2020, 7, 1564. |
| [1] | (f) Kim, S.; Loose, F.; Chirik, P. J. Chem. Rev. 2020, 120, 5637. |
| [2] | (a) Stappen, C. V.; Decamps, L.; Cutsail III, G. E.; Bjornsson, R.; Henthorn, J. T.; Birrell, J. A.; DeBeer, S. Chem. Rev. 2020, 120, 5005. |
| [2] | (b) Kang, W.; Lee, C. C.; Jasniewski, A. J.; Ribbe, M. W.; Hu, Y. Science 2020, 368, 1381. |
| [3] | (a) Coric, I.; Mercado, B. Q.; Bill, E.; Vinyard, D. J.; Holland, P. L. Nature 2015, 526, 96. |
| [3] | (b) Creutz, S. E.; Peters, J. C. J. Am. Chem. Soc. 2015, 137, 7310. |
| [3] | (c) Le, L. N. V.; Bailey, G. A.; Scott, A. G.; Agapie, T. Proc. Natl. Acad. Sci. U. S. A. 2021, 118, e2109241118. |
| [3] | (d) McSkimming, A.; Suess, D. L. M. Nat. Chem. 2021, 13, 666. |
| [3] | (e) Zhang, Y.; Zhao, J.; Yang, D.; Wang, B.; Zhou, Y.; Wang, J.; Chen, H.; Mei, T.; Ye, S.; Qu, J. Nat. Chem. 2022, 14, 46. |
| [3] | (f) Ouyang, Z.; Cheng, J.; Li, L.; Bao, X.; Deng, L. Chem. -Eur. J. 2016, 22, 14162. |
| [3] | (g) Fan, Y.; Cheng, J.; Gao, Y.; Shi, M.; Deng, L. Acta Chim. Sinica 2018, 76, 445. (in Chinese) |
| [3] | (凡一明, 程骏, 高亚飞, 施敏, 邓亮, 化学学报, 2018, 76, 445.) |
| [4] | (a) Beinert, H.; Holm, R. H.; Munck, E. Science 1997, 277, 653. |
| [4] | (b) Venkateswara Rao, P.; Holm, R. H. Chem. Rev. 2004, 104, 527; |
| [4] | (c) Lee, S. C.; Lo, W.; Holm, R. H. Chem. Rev. 2014, 114, 3579. |
| [5] | Buijse, M. A.; Baerends, E. J. J. Chem. Phys. 1990, 93, 4129. |
| [6] | Li, Y.; Rauchfuss, T. B. Chem. Rev. 2016, 116, 7043. |
| [7] | Ellison, J. J.; Ruhlandt-Senge, K.; Power, P. P. Angew. Chem. Int. Ed. 1994, 33, 1178. |
| [8] | Ouyang, Z.; Du, J.; Wang, L.; Kneebone, J. L.; Neidig, M. L.; Deng, L. Inorg. Chem. 2015, 54, 8808. |
| [9] | Bedford, R. B.; Brenner, P. B.; Carter, E.; Clifton, J.; Cogswell, P. M.; Gower, N. J.; Haddow, M. F.; Harvey, J. N.; Kehl, J. A.; Murphy, D. M.; Neeve, E. C.; Neidig, M. L.; Nunn, J.; Snyder, B. E. R.; Taylor, J. Organometallics 2014, 33, 5767. |
| [10] | Mock, M. T.; Popescu, C. V.; Yap, G. P.; Dougherty, W. G.; Riordan, C. G. Inorg. Chem. 2008, 47, 1889. |
| [11] | Rodriguez, M. M.; Stubbert, B. D.; Scarborough, C. C.; Brennessel, W. W.; Bill, E.; Holland, P. L. Angew. Chem. Int. Ed. 2012, 51, 8247. |
| [12] | (a) Tanabe, Y.; Nishibayashi, Y. Coord. Chem. Rev. 2019, 389, 73. |
| [12] | (b) Yin, J.; Li, J.; Wang, G.; Yin, Z.; Zhang, W.; Xi, Z. J. Am. Chem. Soc. 2019, 141, 4241. |
| [12] | (c) Zhong, M.; Cui, X.; Wu, B.; Wang, G.; Zhang, W.; Wei, J.; Zhao, L.; Xi, Z. CCS Chem. 2022, 4, 532. |
| [13] | Ferreira, R. B.; Cook, B. J.; Knight, B. J.; Catalano, V. J.; García-Serres, R.; Murray, L. J. ACS Catal. 2018, 8, 7208. |
| [14] | Liang, Q.; DeMuth, J. C.; Radovic, A.; Wolford, N. J.; Neidig, M. L.; Song, D. Inorg. Chem. 2021, 60, 13811. |
/
| 〈 |
|
〉 |