

Rb2MGe3S8 (M=Zn, Cd): Non-Centrosymmetry Transformation Led by Structure Change of [MGe3S8]2- Unit※
Received date: 2022-01-12
Online published: 2022-02-17
Supported by
National Natural Science Foundation of China(22075283); National Natural Science Foundation of China(22175172); National Natural Science Foundation of China(92161125); Youth Innovation Promotion Association of Chinese Academy of Sciences(2020303); Youth Innovation Promotion Association of Chinese Academy of Sciences(2021300)
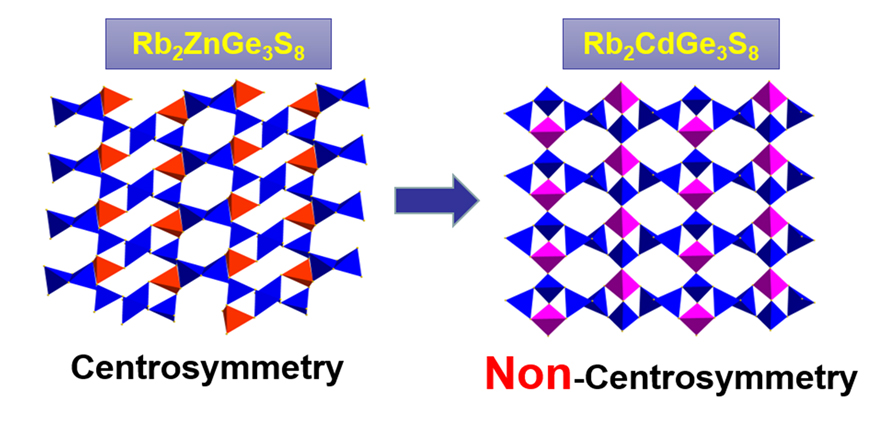
Infrared nonlinear (IR NLO) optical crystals have an essential position in military and civilian fields because of their ability to convert lasers from near infrared (NIR) to mid/far infrared (MIR/FIR). In this work, two alkali-metal chalcogenides, Rb2MGe3S8 [M=Zn (1), Cd (2)], were successfully synthesized by high-temperature solid-state reactions. Both compounds feature a two-dimensional layered structure and have a large optical band-gap, the experimental band-gap of 1 and 2 are 3.24 eV and 3.16 eV, respectively. Compound 1 belongs to the centrosymmetric group P-1, while 2 belongs to the non-centrosymmetric space group P2(1)2(1)2(1) and exhibits obvious NLO effect, which is comparable to that of KH2PO4 (KDP) (@1064 nm) at the particle size of 50~75 μm. Particle-size dependent NLO response measurements indicated that 2 is non-phase-matchable. Compound 2 exhibits a high laser-induced damage threshold of 16.6×AGS at 1064 nm. Through the analysis of the crystal structures of these two compounds, the reason why their formulas have the same stoichiometric ratio but symmetries are different is the structure change of basic building unit [MGe3S8]2– in 1 and 2. All M atoms in both compounds are coordinated by four S atoms to form MS4 tetrahedra. In each [CdGe3S8]2– unit of 2, three S atoms bonded to the Cd atom are also bonded to all Ge atoms in that unit, that is to say, each CdS4 tetrahedron is connected to the other three GeS4 tetrahedra by sharing S vertices. Unlike the coordination manner in the [CdGe3S8]2– unit of 2, there are only two S atoms bonded to both Zn and Ge atoms in [ZnGe3S8]2– unit of 1. This structure change of [MGe3S8]2– unit eventually led to the non-centrosymmetric transformation. What’s more, to get insight into the origin of NLO effect of 2, theoretical calculations of electronic band structure and NLO susceptibility were performed based on density functional theory.
Xiandan Chai , Wenfa Chen , Qiunan Yan , Binwen Liu , Xiaoming Jiang , Guocong Guo . Rb2MGe3S8 (M=Zn, Cd): Non-Centrosymmetry Transformation Led by Structure Change of [MGe3S8]2- Unit※[J]. Acta Chimica Sinica, 2022 , 80(5) : 633 -639 . DOI: 10.6023/A22010020
| [1] | Boyd, G.; Kasper, H.; Mcfee, J. IEEE J. Quantum Elect. 1971, 7, 563. |
| [2] | Byer, R. L.; Choy, M. M.; Herbst, R. L.; Chemla, D. S.; Feigelson, R. S. Appl. Phys. Lett. 1974, 24, 65. |
| [3] | Boyd, G. D.; Buehler, E.; Storz, F. G. Appl. Phys. Lett. 1971, 18, 30. |
| [4] | Yang, Z.-H.; Pan, S.-L. J. Synthetic Cryst. 2019, 48, 17. (in Chinese) |
| [4] | (杨志华, 潘世烈, 人工晶体学报, 2019, 48, 17.) |
| [5] | Gong, P.-F.; Liang, F.; Kang, L.; Chen, X.-G.; Qin, J.-G; Wu, Y.-C.; Lin, Z.-S. Coord. Chem. Rev. 2019, 380, 83. |
| [6] | Pan, Y.; Guo, S.-P.; Liu, B.-W.; Xue, H.-G.; Guo, G.-C. Coord. Chem. Rev. 2018, 374, 464. |
| [7] | Jiang, X.-M.; Deng, S.-Q.; Whangbo, M. H.; Guo, G.-C. Natl. Sci. Rev. 2022, DOI: 10.1093/nsr/nwac017. |
| [8] | Zhao, S.-G.; Gong, P.-F.; Luo, S.-Y.; Bai, L.; Lin, Z.-S.; Tang, Y.-Y.; Zhou, Y.-L.; Hong, M.-C.; Luo, J.-H. Angew. Chem., Int. Ed. 2015, 54, 4217. |
| [9] | Wu, K.; Yang, Z.-H.; Pan, S.-L. Angew. Chem., Int. Ed. 2016, 55, 6713. |
| [10] | Isaenko, L.; Yelisseyev, A.; Lobanov, S.; Panich, A.; Vedenyapin, V.; Smirnova, J.; Petrov, V.; Zondy, J. J.; Knippels, G. MRS Online Proc. Libr. 2001, 692, DOI: 10.1557/PROC-692-H9.7.1 |
| [11] | Wu, K.; Zhang, B.-B.; Yang, Z.-H.; Pan, S.-L. J. Am. Chem. Soc. 2017, 139, 14885. |
| [12] | Li, M.-Y.; Li, B.-X.; Lin, H.; Shi, Y.-F.; Ma, Z.-J.; Wu, L.-M.; Wu, X.-T.; Zhu, Q.-L. Inorg. Chem. 2018, 57, 8730. |
| [13] | Wu, K.; Yang, Z.-H.; Pan, S.-L. Chem. Mater. 2016, 28, 2795. |
| [14] | Guo, S.-P.; Chi, Y.; Guo, G.-C. Coord. Chem. Rev. 2017, 335, 44. |
| [15] | Hu, X.-N.; Xiong, L.; Wu, L.-M. Cryst. Growth Des. 2018, 18, 3124. |
| [16] | Luo, X.-Y.; Fei, L.; Zhou, M.-L.; Guo, Y.-W.; Li, Z.; Lin, Z.-S.; Yao, J.-Y.; Wu, Y.-C. Inorg. Chem. 2018, 57, 9446. |
| [17] | Gao, L.-H.; Yang, Y.; Zhang, B.-B.; Wu, X.-W.; Wu, K. Inorg. Chem. 2021, 60, 12573. |
| [18] | Morris, C. D.; Li, H.; Jin, H.; Malliaksa, C. D.; Peters, J. A.; Trikalitis, P. N.; Freeman, A. J.; Wessels, B. W.; Kanatzidis, M. G. Chem. Mater. 2013, 25, 3344. |
| [19] | Yang, L.-Q.; Ye, R.; Jiang, X.-M.; Liu, B.-W.; Zeng, H.-Y.; Guo, G.-C. J. Mater. Chem. C 2020, 8, 3688. |
| [20] | Rigaku Oxford Diffraction, CrysAlisPro Software System, version v40.67a, Rigaku Corporation: Oxford, UK, 2019. |
| [21] | Sheldrick, G. M. SHELXS-97: Program for X-ray Crystal Structure Solution, University of Go?ttingen, Germany, 1997. |
| [22] | Spek, A. L.; Platon, A. Multipurpose Crystallographic Tool, Utrecht University, Utrecht, Netherlands, 2005. |
| [23] | Kortu?m, G. Reflectance Spectroscopy, Springer, New York, 1969. |
| [24] | Zhou, H.-M.; Wu, L.-M. Chin. Sci. Bull. 2019, 9, 879. (in Chinese) |
| [24] | (周慧敏, 吴立明, 科学通报, 2019, 9, 879.) |
| [25] | Xie, H.; Fang, S.-H.; Zhao, H.; Xu, X.-L.; Ye, N.; Zhuang, W. RSC Adv. 2019, 9, 35771. |
| [26] | Kurtz, S. K.; Perry, T. T. J. Appl. Phys. 1968, 39, 3798. |
| [27] | Clark, S. J.; Segall, M. D.; Pickard, C. J.; Hasnip, P. J.; Probert, M. J.; Refson, K.; Payne, M. C. Z. Krist.-Cryst. Mater. 2005, 220, 5. |
| [28] | Payne, M. C.; Teter, M. P.; Allan, D. C.; Arias, T. A.; Joannopoulos, J. D. Rev. Mod. Phys. 1992, 64, 1045. |
| [29] | Perdew, J. P.; Chevary, J. A.; Vosko, S. H.; Jackson, K. A.; Pederson, M. R.; Singh, D. J.; Fiolhais, C. Phys. Rev. B Condens. Matter 1993, 46, 6671. |
| [30] | Sharma, S.; Ambrosch-Draxl, C. Phys. Scr. 2004, T109, 128. |
| [31] | Laksari, S.; Chahed, A.; Abbouni, N.; Benhelal, O.; Abbar, B. Comput. Mater. Sci. 2007, 38, 223. |
| [32] | Mo, S. D.; Ching, W. Y. Phys. Rev. B Condens. Matter 1995, 51, 3023. |
/
| 〈 |
|
〉 |