

Efficient Chemical Synthesis and Oxidative Folding Studies of Scorpion Toxin Peptide WaTx
Received date: 2021-12-24
Online published: 2022-02-17
Supported by
National Natural Science Foundation of China(21807063); National Natural Science Foundation of China(82003647); National Natural Science Foundation of China(22177058); National Natural Science Foundation of China(81870653)
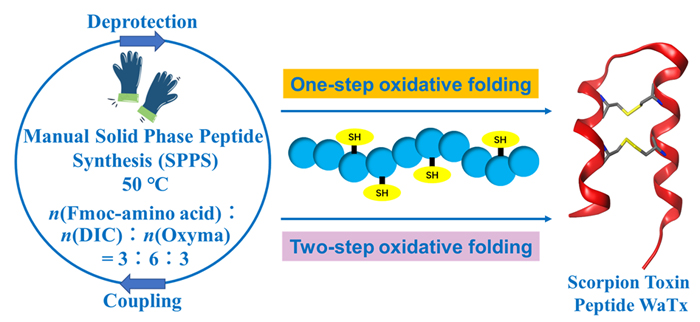
Coupling or condensation reagents that could be used to promote the condensation of carboxylic acids with amines to furnish amide bonds play crucial roles in solid phase peptide synthesis (SPPS) of peptides and peptide-based derivatives. Compared with traditional coupling reagents used in SPPS such as 1-hydroxy-7-azabenzotriazole (HOAt) and 1-hydroxybenzotriazole (HOBt), the novel N,N'-diisopropylcarbodiimide (DIC)/ethyl 2-cyano-2-(hydroxyimino) acetate (Oxyma) condensation system has advantages of inexpensiveness, safety, high coupling efficiency, low racemic rate, and compatibility with manual and automatic peptide synthesis represented by microwave-assisted SPPS. However, the effects of different reaction temperatures (e.g. 28, 50, and 75 ℃) on the coupling efficiency of DIC/Oxyma and on the easily oxidized amino acids such as methionine (Met) remain to be further investigated. The transient receptor potential ankyrin 1 (TRPA1) channel plays an important role in temperature perception, auditory perception, and inflammatory pain. As a novel non-covalently TRPA1-specific agonist, Wasabi Receptor Toxin (WaTx) that consists of 33 amino acid residues and two pairs of disulfide bonds is considered as an important molecular tool to study the opening mechanism and function of TRPA1. In this study, 2-(6-chloro-1H-benzotriazole-1-yl)-1,1,3,3-tetramethylaminiumhexa-fluorophosphate (HCTU)/N,N'-diisopropyl- ethylamine (DIEA) and DIC/Oxyma condensation systems were separately utilized to explore the synthetic efficiency of linear WaTx and the oxidative degree of Met residues at different temperatures. The robustness of DIC/Oxyma condensation system was validated by the rapid manual synthesis of linear WaTx. The one-step and two-step oxidative folding strategies were separately applied for the construction of two pairs of disulfide bridges, affording the active WaTx, which was further confirmed by circular dichroism and calcium fluorescence assay. In this study, a moderate, efficient synthesis and renaturation folding method of WaTx was established. Moreover, the effects of different reaction temperatures on the synthetic efficiency of DIC/Oxyma and on amino acid residues oxidization were compared for the first time. From the aspects of reaction efficiency (represented by the timescale of the overall Fmoc deprotection-washing-amino acid coupling SPPS cycle), amino acid residues oxidization and synthetic cost, n(Fmoc-amino acid):n(DIC):n(Oxyma)=3:6:3 under 50 ℃ should be the ideal reaction condition. This work provides both an imperative complement for SPPS and a particularly useful strategy for the manual efficient synthesis of disulfide-containing peptides with easily oxidized groups.
Hao Yin , Xitong Chen , Xingyan Fu , Yannan Ma , Yimei Xu , Te Zhang , Shuai Liang , Shanshan Du , Yunkun Qi , Kewei Wang . Efficient Chemical Synthesis and Oxidative Folding Studies of Scorpion Toxin Peptide WaTx[J]. Acta Chimica Sinica, 2022 , 80(4) : 444 -452 . DOI: 10.6023/A21120580
| [1] | (a) Liu, T.; Xu, S. L.; Zhao, J. F. Chin. J. Org. Chem. 2021, 41, 873. (in Chinese) |
| [1] | (刘涛, 许泗林, 赵军锋, 有机化学, 2021, 41, 873.) |
| [1] | (b) Chang, H. N.; Liu, B. Y.; Qi, Y. K.; Zhou, Y.; Chen, Y. P.; Pan, K. M.; Li, W. W.; Zhou, X. M.; Ma, W. W.; Fu, C. Y.; Qi, Y. M.; Liu, L.; Gao, Y. F. Angew. Chem., Int. Ed. 2015, 54, 11760. |
| [1] | (c) Guo, Y.; Zhou, P. P.; Zhang, S. Y.; Fan, X. W.; Dou, Y. W.; Shi, X. L. Med ChemComm 2018, 9, 1226. |
| [1] | (d) Zhou, X. M.; Zuo, C.; Li, W. Q.; Shi, W. W.; Zhou, X. W.; Wang, H. F.; Chen, S. M.; Du, J. F.; Chen, G. Y.; Zhai, W. J.; Zhao, W. S.; Wu, Y. H.; Qi, Y. M.; Liu, L.; Gao, Y. F. Angew. Chem., Int. Ed. 2020, 59, 15114. |
| [1] | (e) Song, H.; Liu, C.; Wu, Y. J.; Hu, H. G.; Yan, F. Acta Chim. Sinica 2018, 76, 95. (in Chinese) |
| [1] | (宋慧, 刘超, 吴仪君, 胡宏岗, 阎芳, 化学学报, 2018, 76, 95.) |
| [1] | (f) Cui, T. T.; Chen, J. Y.; Zhao, R.; Guo, Y. Y.; Tang, J. H.; Li, Y. L.; Li, Y. M.; Bierer, D.; Liu, L. Chin. J. Chem. 2021, 39, 2517. |
| [2] | (a) Guo, Y.; Fu, L. L.; Fan, X. W.; Shi, X. L. Chin. J. Org. Chem. 2018, 38, 1267. (in Chinese) |
| [2] | (郭叶, 傅莉莉, 范晓文, 史宣玲, 有机化学, 2018, 38, 1267.) |
| [2] | (b) Qi, Y. K.; Qu, Q.; Bierer, D.; Liu, L. Chem. Asian J. 2020, 15, 2793. |
| [2] | (c) Guo, Y.; Fu, L. L.; Fan, X. W.; Shi, X. L. Chin. Chem. Lett. 2018, 29, 1167. |
| [3] | (a) Muramatsu, W.; Yamamoto, H. J. Am. Chem. Soc. 2021, 143, 6792. |
| [3] | (b) Hu, L.; Xu, S.; Zhao, Z.; Yang, Y.; Peng, Z.; Yang, M.; Wang, C.; Zhao, J. J. Am. Chem. Soc. 2016, 138, 13135. |
| [3] | (c) Jaradat, D. M. M. Amino Acids 2018, 50, 39. |
| [4] | Wang, Z.; Wang, X.; Wang, P.; Zhao, J. J. Am. Chem. Soc. 2021, 143, 10374. |
| [5] | (a) Albericio, F.; El-Faham, A. Org. Process Res. Dev. 2018, 22, 760. |
| [5] | (b) Isidro-Llobet, A.; Kenworthy, M. N.; Mukherjee, S.; Kopach, M. E.; Wegner, K.; Gallou, F.; Smith, A. G.; Roschangar, F. J. Org. Chem. 2019, 84, 4615. |
| [5] | (c) Subiros-Funosas, R.; Prohens, R.; Barbas, R.; El-Faham, A.; Albericio, F. Chem. Eur. J. 2009, 15, 9394. |
| [6] | (a) Wang, F.; Xu, L.; Chu, G.; Shi, J.; Guo, Q. Chin. J. Org. Chem. 2016, 36, 218. (in Chinese) |
| [6] | (王风亮, 许玲, 储国超, 石景, 郭庆祥, 有机化学, 2016, 36, 218.) |
| [6] | (b) Pan, M.; Gao, S.; Zheng, Y.; Tan, X.; Lan, H.; Tan, X.; Sun, D.; Lu, L.; Wang, T.; Zheng, Q.; Huang, Y.; Wang, J.; Liu, L. J. Am. Chem. Soc. 2016, 138, 7429. |
| [6] | (c) Qi, Y.-K.; Si, Y.-Y.; Du, S.-S.; Liang, J.; Wang, K.-W.; Zheng, J.-S. Sci. China Chem. 2019, 62, 299. |
| [6] | (d) Qi, Y. K.; Ai, H. S.; Li, Y. M.; Yan, B. Front. Chem. 2018, 6, 19. |
| [6] | (e) Huang, Y. C.; Guan, C. J.; Tan, X. L.; Chen, C. C.; Guo, Q. X.; Li, Y. M. Org. Biomol. Chem. 2015, 13, 1500. |
| [6] | (f) Ben Haj Salah, K.; Inguimbert, N. Org. Lett. 2014, 16, 1783. |
| [7] | (a) Qu, Q.; Pan, M.; Gao, S.; Zheng, Q. Y.; Yu, Y. Y.; Su, J. C.; Li, X.; Hu, H. G. Adv. Sci. 2018, 5, 1800234. |
| [7] | (b) Qu, Q.; Gao, S.; Wu, F.; Zhang, M. G.; Li, Y.; Zhang, L. H.; Bierer, D.; Tian, C. L.; Zheng, J. S.; Liu, L. Angew. Chem., Int. Ed. 2020, 59, 6037. |
| [7] | (c) Zuo, C.; Shi, W.-W.; Chen, X.-X.; Glatz, M.; Riedl, B.; Flamme, I.; Pook, E.; Wang, J.; Fang, G.-M.; Bierer, D.; Liu, L. Sci. China Chem. 2019, 62, 1371. |
| [8] | Liang, L. J.; Chu, G. C.; Qu, Q.; Zuo, C.; Mao, J.; Zheng, Q.; Chen, J.; Meng, X.; Jing, Y.; Deng, H.; Li, Y. M.; Liu, L. Angew. Chem., Int. Ed. 2021, 60, 17171. |
| [9] | (a) Ma, Y.; Liu, Y.; Wang, J.; Chen, X.; Yin, H.; Chi, Q.; Jia, S.; Du, S.; Qi, Y.; Wang, K. Chin. J. Org. Chem. 2022, 42, 10.6023/cjoc202109003. (in Chinese) |
| [9] | (马艳楠, 刘雅妮, 王金艳, 陈西同, 尹昊, 迟巧娜, 贾世玺, 杜姗姗, 齐昀坤, 王克威, 有机化学, 2022, 42, 10.6023/cjoc202109003.) |
| [9] | (b) Chen, X. T.; Wang, J. Y.; Ma, Y. N.; Dong, L. Y.; Jia, S. X.; Yin, H.; Fu, X. Y.; Du, S. S.; Qi, Y. K.; Wang, K. J. Pept. Sci. 2022, 28, e3368. |
| [10] | Qiao, Z.; Luo, J.; Tang, Y. Q.; Zhou, Q.; Qi, H.; Yin, Z.; Tang, X.; Zhu, W.; Zhang, Y.; Wei, N.; Wang, K. J. Med. Chem. 2021, 64, 16282. |
| [11] | King, J. V. L.; Emrick, J. J.; Kelly, M. J. S.; Herzig, V.; King, G. F.; Medzihradszky, K. F.; Julius, D. Cell 2019, 178, 1362. |
| [12] | (a) Wang, J.; Dong, L.; Liu, Y.; Chen, X.; Ma, Y.; Yin, H.; Du, S.; Qi, Y.; Wang, K. Chin. J. Org. Chem. 2021, 41, 2800. (in Chinese) |
| [12] | (王金艳, 董黎颖, 刘雅妮, 陈西同, 马艳楠, 尹昊, 杜姗姗, 齐昀坤, 王克威, 有机化学, 2021, 41, 2800.) |
| [12] | (b) Qi, Y. K.; He, Q. Q.; Ai, H. S.; Guo, J.; Li, J. B. Chem. Commun. 2017, 53, 4148. |
| [13] | (a) Shi, J.; So, L. Y.; Chen, F.; Liang, J.; Chow, H. Y.; Wong, K. Y.; Wan, S.; Jiang, T.; Yu, R. J. Pept. Sci. 2018, 24, e3087. |
| [13] | (b) Guo, Y.; Sun, D. M.; Wang, F. L.; He, Y.; Liu, L.; Tian, C. L. Angew. Chem., Int. Ed. 2015, 54, 14276. |
| [14] | (a) Zheng, J. S.; Tang, S.; Qi, Y. K.; Wang, Z. P.; Liu, L. Nat. Protoc. 2013, 8, 2483. |
| [14] | (b) Li, J. B.; Qi, Y. K.; He, Q. Q.; Ai, H. S.; Liu, S. L.; Wang, J. X.; Zheng, J. S.; Liu, L.; Tian, C. Cell Res. 2018, 28, 257. |
| [14] | (c) Zheng, J. S.; Liang, J.; Shi, W. W.; Li, Y.; Hu, H. G.; Tian, C. L.; Liu, L. Sci. Bull. 2021, 66, 1542. |
| [15] | Wang, N.; Xie, G.; Liu, C.; Cong, W.; He, S.; Li, Y.; Fan, L.; Hu, H. G. Front. Chem. 2020, 8, 616147. |
/
| 〈 |
|
〉 |