

Rare Earth Ions-Activated Hybrid Assemblies Fluorescent Systems Based on the Layered Lanthanum Hydroxides
Received date: 2021-10-27
Online published: 2021-11-16
Supported by
National Natural Science Foundation of China(51972097)
The layered rare earth hydroxides (LRHs) have recently been paid attention owing to the adjustable rare earth ions in the host and alterable anions in the gallery, which may be applied in adsorption, catalyst, fluorescence and detection fields. However, as far as we know, the studies of rare earth ions-activated layered lanthanum hydroxide (LLaH) fluorescent systems were seldom reported. In this paper, the hybrid assemblies (Eu/Tb)0.1La1.9(OH)5DS•H2O and Ce0.08La1.92(OH)5DS• H2O solid samples were first prepared with Eu(NO3)3, TbCl3, Ce(NO3)3•6H2O and La(NO3)3 solutions by hydrothermal process at 140 ℃ for 12 h, in which the interlayer was pillared with dodecyl sulfonate (DS-) anions. Then, the DS- anions of the as-prepared (Eu/Tb)0.1La1.9(OH)5DS•H2O were exchanged with benzoate (BA-) solution through microwave process, and the hybrid assemblies samples (Eu/Tb)0.1La1.9(OH)5BA•H2O were obtained. The X-ray diffraction (XRD) results indicate that the solid samples have superior crystallization and layered structure, and the interlayer distance is decreased from 3.2 nm to 1.9 nm after the ion-exchange reaction. The photoluminescence excitation spectra show that the excitation intensities in the ultraviolet (UV) region (≈280 nm) for (Eu/Tb)0.1La1.9(OH)5BA•H2O are much stronger than those for (Eu/Tb)0.1La1.9- (OH)5DS•H2O due to the strong absorption peak from BA- anion. Excited with 280 nm, the emission spectra exhibit the characteristic emission transitions of Eu3+ or Tb3+ ions, and the intensities of (Eu/Tb)0.1La1.9(OH)5BA•H2O are more than tenfold of (Eu/Tb)0.1La1.9(OH)5DS•H2O because of efficient sensitization effect of BA-. On the other hand, the (Eu/Tb)0.1La1.9(OH)5BA•H2O and Ce0.08La1.92(OH)5DS•H2O hybrid assemblies were exfoliated to colloidal solutions through ultrasound and centrifugation process in formamide solvent. It has been observed that the fluorescent color can be adjusted by mixing the colloid solutions of Eu0.1La1.9(OH)5BA•H2O and Tb0.1La1.9(OH)5BA•H2O in different volume ratios. Moreover, the fluorescent color can be changed with the three colloid components by varying the excitation wavelength. In particular, the white light can be achieved as excited with 280—290 nm UV light, exhibiting supreme photofunctional performances. The investigation will promote the effective combination of rare earth luminescence with layered materials.
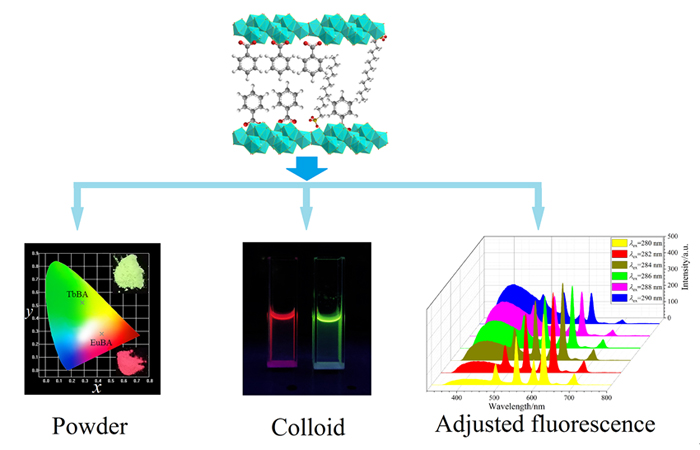
Key words: layered hydroxide; rare earth; fluorescence; luminescent colloid; ion-exchange
Xiangyu Zuo , Yifei Xu , Shikao Shi . Rare Earth Ions-Activated Hybrid Assemblies Fluorescent Systems Based on the Layered Lanthanum Hydroxides[J]. Acta Chimica Sinica, 2022 , 80(2) : 133 -140 . DOI: 10.6023/A21100484
| [1] | Liu, J.; Kaczmarek, A. M.; Deun, R. V. Chem. Soc. Rev. 2018, 47, 7225. |
| [2] | Qin, X.; Liu, X. W.; Huang, W.; Bettinelli, M.; Liu, X. G. Chem. Rev. 2017, 117, 4488. |
| [3] | Yang, Y. S.; Wang, K. Z.; Yan, D. P. ACS Appl. Mater. Interfaces 2017, 9, 17399. |
| [4] | Yang, X. G.; Lin, X. Q.; Zhao, Y. B.; Zhao, Y. S.; Yan, D. P. Angew. Chem., Int. Ed. 2017, 56, 7853. |
| [5] | Yang, Y.-S.; Wang, K.-Z.; Zhao, Z.; Yan, D.-P. J. Chin. Soc. Rare Earths 2021, 39, 1. (in Chinese) |
| [5] | ( 杨永晟, 王克志, 赵震, 闫东鹏, 中国稀土学报, 2021, 39, 1.) |
| [6] | Gao, R.; Zhao, M. J.; Guan, Y.; Fang, X. Y.; Li, X. H.; Yan, D. P. J. Mater. Chem. C 2014, 2, 9579. |
| [7] | Gao, R.; Kodaimati, M. S.; Yan, D. P. Chem. Soc. Rev. 2021, 50, 5564. |
| [8] | Yapryntsev, A.; Abdusatorov, B.; Yakushev, I.; Svetogorov, R.; Gavrikov, A.; Rodina, A.; Fatyushina, Y.; Baranchikov, A.; Zubavichus, Y.; Ivanov, V. Dalton Trans. 2019, 48, 6111. |
| [9] | Jung, H.; Kim, H.; Byeon, S. H. ACS Appl. Mater. Interfaces 2018, 10, 43112. |
| [10] | Shao, B. Y.; Zhang, X. B.; Sang, S.; Guo, A. P.; Cui, F. M.; Yang, X. J. Eur. Polym. J. 2021, 147, 110324. |
| [11] | Kim, H.; Lee, B. I.; Jeong, H.; Byeon, S. H. J. Mater. Chem. C 2015, 3, 7437. |
| [12] | Jeong, H.; Lee, B. I.; Byeon, S. H. ACS Appl. Mater. Interfaces 2016, 8, 10946 |
| [13] | Wang, B.; Xia, J. F.; Zhou, G. H.; Li, X.; Dai, M. T.; Jiang, D. Y.; Li, Q. RSC Adv. 2020, 10, 37500. |
| [14] | Shao, B. Y.; Zhang, X. B.; Wang, X. Y.; Cui, F. M.; Yang, X. J. Opt. Mater. 2020, 100, 109597. |
| [15] | Pereira, C. C. L.; Lima, J. C.; Moro, A. J.; Monteiro, B. Appl. Clay Sci. 2017, 146, 216. |
| [16] | Sokolov, M. R.; Enakieva, Y. Y.; Yapryntsev, A. D.; Shiryaev, A. A.; Zvyagina, A. I.; Kalinina, M. A. Adv. Funct. Mater. 2020, 30, 2000681. |
| [17] | Gu, Q. Y.; Li, J. Y.; Ji, L. S.; Ju, R. J.; Jin, H. B.; Zhang, R. Y. Front. Mater. Sci. 2020, 14, 488. |
| [18] | Chen, Y. F.; Zhang, Y. J.; Zhang, J. W.; Wang, L. Luminescence 2020, 35, 1125. |
| [19] | Jeon, H. G.; Kim, H.; Byeon, S. H. Chem. Eng. J. 2021, 405, 126675. |
| [20] | Wu, L. Y.; Chen, G. M.; Li, Z. B. Small 2017, 13, 1604070. |
| [21] | Wu, L. Y.; Gao, C. Y.; Li, Z. B.; Chen, G. M. J. Mater. Chem. C 2017, 5, 5207. |
| [22] | Su, F. F.; Guo, R.; Yu, Z. H.; Li, J.; Liang, Z. P.; Shi, K. R.; Ma, S. L.; Sun, G. B.; Li, H. F. Dalton Trans. 2018, 47, 5380. |
| [23] | Zhu, Q.; Li, J. G.; Zhi, C.; Ma, R.; Sasaki, T.; Xu, J. X.; Liu, C. H.; Li, X. D.; Sun, X. D.; Sakka, Y. J. Mater. Chem. 2011, 21, 6903. |
| [24] | McIntyre, L. J.; Jackson, L. K.; Fogg, A. M. J. Phys. Chem. Solids 2008, 69, 1070. |
| [25] | Jeon, H. G.; Kim, H.; Byeon, S. H. Adv. Mater. Interfaces 2019, 1901385. |
| [26] | Liu, W. D.; Zhang, J.; Yin, X. B.; He, X. Y.; Wang, X. P.; Wei, Y. Z. Mater. Chem. Phys. 2021, 266, 124540. |
| [27] | Ma, H.; Li, X.-X.; Sun, Y.-H.; Wang, J.; Chu, N.-K.; Ma, S.-L. J. Beijing Norm. Univ. (Nat. Sci.) 2011, 47, 607. (in Chinese) |
| [27] | ( 马辉, 李新新, 孙亚红, 王娟, 楚楠凯, 马淑兰, 北京师范大学学报(自然科学版), 2011, 47, 607.) |
| [28] | Xu, J.-J.; Zhang, Q.-M.; Jin, Z.; Chu, H.-B.; Li, Y. Chin. J. Inorg. Chem. 2006, 22, 1411. (in Chinese) |
| [28] | ( 许军舰, 张庆敏, 金钟, 褚海斌, 李彦, 无机化学学报, 2006, 22, 1411.) |
| [29] | Hou, X.-H.; Xu, G.; Wei, X.; Han, G.-R. Rare Met. Mater. Eng. 2008, 37, 437. (in Chinese) |
| [29] | ( 侯晓红, 徐刚, 魏晓, 韩高荣, 稀有金属材料与工程, 2008, 37, 437.) |
| [30] | Anchalee, W.; Franziska, S.; Warner, J. H.; Dermot, O. J. Mater. Chem. 2012, 22, 7751. |
| [31] | Zhang, K.-L.; Yuan, J.-B.; Yuan, L.-J.; Sun, J.-T. Acta Chim. Sinica 2000, 58, 144. (in Chinese) |
| [31] | ( 张克立, 袁继兵, 袁良杰, 孙聚堂, 化学学报, 2000, 58, 144.) |
| [32] | Yuan, L.; Sun, J.; Zhang, K. Spectrochim. Acta, Part A 2003, 59, 729. |
| [33] | Utochnikova, V. V.; Kuzmina, N. P. Russ. J. Coord. Chem. 2016, 42, 679. |
| [34] | Zhong, S. L.; Zhang, L. F.; Jiang, J. W.; Lv, Y. H.; Xu, R.; Xu, A. W.; Wang, S. P. CrystEngComm 2011, 13, 4151. |
| [35] | Liu, L.; Yu, J. J.; Shi, S. K.; Wang, J. Y.; Song, H. H.; Zhang, R. K.; Fu, L. S. J. Rare Earths, https://doi.org/10.1016/j.jre.2021.07.011. |
/
| 〈 |
|
〉 |