

K+-Site Ce-Doped Jarosite for Phosphate Adsorption: a Mechanism Study※
Received date: 2021-12-30
Online published: 2022-03-04
Supported by
Youth Innovation Promotion Association CAS(2021302); Youth Innovation Promotion Association CAS(2021305); FJIRSM&IUE Joint Research Fund(RHZX-2019-003)
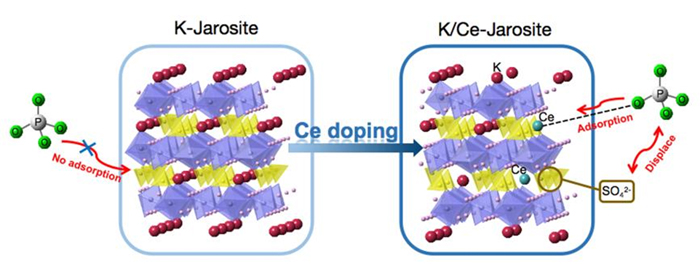
Jarosite is a common iron-containing mineral. Researchers have studied its application for removing aqueous pollutants, such as Cr(VI) and As(V). Surprisingly, it shows adsorption for arsenates, but little for the structurally similar phosphate ions. In this study, we prepare cerium doped jarosite and prove the successful doping of cerium at the K+ site by X-ray diffraction (XRD), inductively coupled plasma-optical emission spectrometry (ICP-OES), energy dispersive spectroscopy (EDS), and X-ray photoelectron spectroscopy (XPS). Phosphorus adsorption experiments show that the small amount of cerium doping (Ce content: 8.75×10-5 mol/g) significantly improves the phosphate adsorption of jarosite, from 1.69 mg/g to 29.33 mg/g (pH=7, 24 h). The phosphate adsorption of Ce-doped jarosite exhibits good pH stability (from pH=3 to pH=11) and excellent selectivity, which is capable of maintaining more than 91% of its adsorption capacity in the presence of various competing anions, such as HCO3-, CO32-, humic acid anion, SO42-, NO3-, and SiO32-. Further analysis reveals that the adsorption process obeys the pseudo-second order kinetic model while the adsorption isotherms represent the Freundlich isotherm. The analysis indicates that the adsorption may be a chemical adsorption process that is easy to proceed. To explore the mechanism of adsorption enhancement, we first characterize the Zeta-potential of the pure jarosite and Ce-doped jarosite. The result indicates similarity of the surface potential between the two samples, which rules out the electrostatic adsorption mechanism. Next, based on the result of anion exchange chromatography, we confirm that the cerium doping greatly increases the exchange between the sulfate groups in jarosite and the phosphate groups in solution, from 2.85 mg/g to 24.90 mg/g. Finally, XPS high-resolution spectroscopy reveals that the chemical environment of Ce changes after the phosphate adsorption, likely indicating the formation of Ce—O—P chemical bonds to achieve specific chemisorption. These results may provide insights for the modification and application of jarosite, as a new adsorbent material for treating phosphorus rich wastewater.
Key words: jarosite; rare-earth doping; adsorption; phosphate pollutant; cerium
Junrui Liu , Jinglin Chen , Jie Yang , Xiaofeng Xu , Ruonan Li , You-Gui Huang , Shaohua Chen , Xin Ye , Wei Wang . K+-Site Ce-Doped Jarosite for Phosphate Adsorption: a Mechanism Study※[J]. Acta Chimica Sinica, 2022 , 80(4) : 476 -484 . DOI: 10.6023/A21120603
| [1] | Strayer, D. L.; Dudgeon, D. J. N. Am. Benthol. Soc. 2010, 29, 344. |
| [2] | Yang, X. H.; Cai, J. B.; Chen, L. H.; Cao, X.; Liu, H. Z.; Liu, M. X.; Chem. Eng. J. 2021, 425, 130623. |
| [3] | Zhang, Z.; Bi, X.; Li, X. T.; Zhao, Q. C.; Chen, H. H. RSC Adv. 2018, 8, 33583. |
| [4] | Eskandarpour, A.; Sassa, K.; Bando, Y.; Okido, M.; Asai, S. Mater. Trans. 2006, 47, 1837. |
| [5] | Wang, Y.; Wang, J. J.; Li, P.; Qin, H. B.; Liang, J. J.; Fan, Q. H. Environ. Technol. Innovation 2021, 23, 101615. |
| [6] | Elwood Madded, M. E.; Bodnar, R. J.; Rimstidt, J. D. Nature 2004, 431, 821. |
| [7] | Dutrizac, J. E.; Jambor, J. L. Rev. Mineral. Geochem. 2000, 40, 405. |
| [8] | Jin, X. H.; Li, X. F.; Guo, C.; Jiang, M. G.; Yao, Q.; Lu, G. N.; Dang, Z. Sci. Total Environ. 2020, 719, 137311. |
| [9] | Karimian, N.; Johnston, S. G.; Burton, E. D. Environ. Sci. Technol. 2017, 51, 4259. |
| [10] | Fan, C.; Guo, C. L.; Zeng, Y. F.; Tu, Z. H.; Ji, Y. P.; Reinfelder, J. R.; Chen, M. Q.; Huang, W. L.; Lu, G. N.; Yi, X. Y.; Dang, Z. Chemosphere 2019, 222, 945. |
| [11] | Tang, Y. G.; Xie, Y. Y.; Lu, G. N.; Ye, H.; Dang, Z.; Wen, Z. N.; Tao, X. Q.; Xie, C. S.; Yi, X. Y. Chemosphere 2020, 255, 126938. |
| [12] | Li, H.; Wang, N. N.; Xiao, T. F.; Zhang, X. T.; Wang, J. Q.; Tang, J. F.; Kong, Q. N.; Fu, C. B.; Quan, H. B. Chemosphere 2021, 285, 131525. |
| [13] | Dutrizac, J. E.; Chen, T. T. Hydrometallurgy 2010, 102, 5. |
| [14] | Mayakaduwage, S.; Mosley, L. M.; Marschner, P. Geoderma 2020, 371, 114359. |
| [15] | Liu, X. W.; Byrne, R. H. Geochim. Cosmochim. Acta 1997, 61, 1625. |
| [16] | Gu, W.; Xie, Q.; Xing, M. C.; Wu, D. Y. Chem. Eng. Res. Des. 2017, 117, 706. |
| [17] | Hassan, M. H.; Stanton, R.; Secora, J.; Trivedi, D. J.; Andreescu, S. ACS Appl. Mater. Interfaces 2020, 12, 52788. |
| [18] | Neale, Z. G.; Barta, M.; Cao, G. Z. ACS Appl. Energ. Mater. 2021, 4, 2248. |
| [19] | Shannon, R. D. Acta Cryst. 1976, 32, 751. |
| [20] | De Simone, C. A.; Mascarenhas, Y. P.; Svisero, D. P. Rev. Bras. Geocienc. 1985, 15, 164. |
| [21] | Spratt, H. J.; Rintoul, L.; Avdeev, M.; Martens, W. N. Am. Mineral. 2013, 98, 1633. |
| [22] | Grohol, D.; Huang, Q. Z.; Toby, B. H.; Lynn, J. W.; Lee, Y. S.; Nocera, D. G. Phys. Rev. B 2003, 68, 94404. |
| [23] | Grohol, D.; Nocera, D. G. J. Am. Chem. Soc. 2002, 124, 2640. |
| [24] | Basciano, L. C. Ph.D. Dissertation, Queen's University, Kingston, 2008. |
| [25] | Labib, S.; Abdelaal, S.; Abdelhady, A. M.; Elmaghraby, E. K. Mater. Chem. Phys. 2020, 256, 123654. |
| [26] | Basciano, L. C.; Peterson, R. C. Am. Mineral. 2008, 93, 853. |
| [27] | Hernández-Lazcano, E.; Cerecedo-Sáenz, E.; Hernández-Ávila, J.; Toro, N.; Karthik, T. V. K.; Mendoza-Anaya, D.; Fernández-García, M. E.; Rodríguez-Lugo, V.; Salinas-Rodríguez, E. Minerals 2021, 11, 80. |
| [28] | Zhang, Y.; Xi, X. Q.; Xu, S. N.; Zhou, J. C.; Zhou, J. J.; Xu, Q. H.; Shen, H. Y. Acta Chim. Sinica 2012, 70, 1839. (in Chinese) |
| [28] | (张蕴, 奚晓青, 许姗妮, 周俊晨, 周津金, 徐启宏, 沈昊宇, 化学学报, 2021, 70, 1839.) |
| [29] | Xie, J.; Wang, Z.; Fang, D.; Li, C. J.; Wu, D. Y. J. Colloid Interface Sci. 2014, 423, 13. |
| [30] | Trueman, A. M.; Fitzpatrick, R. W.; Mosley, L. M.; McLaughlin, M. J. Chem. Geol. 2021, 561, 120034. |
| [31] | Xu, R.; Zhang, M. Y.; Mortimer, R. J.; Pan, G. Environ. Sci. Technol. 2017, 51, 3418. |
| [32] | Ho, Y. S. Scientometrics 2004, 59, 171. |
| [33] | Ho, Y. S.; McKay, G. Process Biochem. 1999, 34, 451. |
| [34] | Mohan, D.; Singh, K. P.; Singh, V. K. J. Hazard. Mater. 2006, 135, 280. |
| [35] | Ugurlu, M.; Kula, I.; Hamdi Karaoglu, M.; Arslan, Y. Environ. Prog. Sustainable Energy 2009, 28, 547. |
| [36] | Bai, Z. A.; Chen, R. X.; Pang, H. W.; Wang, X. X.; Song, G.; Yu, S. J. Acta Chim. Sinica 2021, 79, 1265. (in Chinese) |
| [36] | (白子昂, 陈瑞兴, 庞宏伟, 王祥学, 宋刚, 于淑君, 化学学报, 2021, 79, 1265.) |
| [37] | Yang, A. L. Rare Met. Mater. Eng. 2018, 47, 1583. (in Chinese) |
| [37] | (杨爱丽, 稀有金属材料, 2018, 47, 1583.) |
| [38] | Haghseresht, F.; Lu, G. Q. Energ Fuels 1998, 12, 1100. |
| [39] | Wang, G. Z.; Zeng, W.; Li, S. S. Environ. Sci. 2021, 42, 4815. (in Chinese) |
| [39] | (王光泽, 曾薇, 李帅帅, 环境科学, 2021, 42, 4815.) |
| [40] | Wang, F.; Liao, Q. L.; Chen, K. R.; Pan, S. Q.; Lu, M. W. J. Non-Cryst. Solids 2015, 409, 76. |
| [41] | Pemba-Mabiala, J. M.; Lenzi, M.; Lenzi, J.; Lebugle, A. Surf. Interface Anal. 1990, 15, 633. |
| [42] | Mao, D. S.; Luo, Z. H.; Li, Z. Y.; Hong, L.; Qu, R. F.; Wang, J. Q.; Jiang, L. J. Mol. Catal. (China) 2018, 32, 315. (in Chinese) |
| [42] | (冒德寿, 罗子豪, 李智宇, 洪鎏, 曲荣芬, 王家强, 姜亮, 分子催化, 2018, 32, 315.) |
| [43] | Kim, Y. J.; Wolf, A. S.; Becker, U. Geochim. Cosmochim. Acta 2019, 248, 138. |
| [44] | Qi, P. F.; Pichler, T. J. Hazard. Mater. 2017, 330, 142. |
| [45] | Chen, K.; Jin, X. H.; Guo, C. L.; He, C. C.; Zhang, Y. Y.; Gao, K.; Lu, G. N.; Dang, Z. Chem. Geol. 2021, 579, 120338. |
| [46] | Derycke, V.; Kongolo, M.; Benzaazoua, M.; Mallet, M.; Barrès, O.; De Donato, P.; Bussière, B.; Mermillod-Blondin, R. Int. J. Miner. Process. 2013, 118, 1. |
| [47] | Neal, A. L.; Techkarnjanaruk, S.; Dohnalkova, A.; Mccready, D.; Peyton, B. M.; Geesey, G. G. Geochim. Cosmochim. Acta 2001, 65, 223. |
| [48] | Siriwardane, R. V.; Cook, J. M. J. Colloid Interface Sci. 1986, 114, 525. |
| [49] | Cao, L. N.; Chen, B. H.; Gou, X. Y.; Zou, Q. Geol. J. Chin. Univ. 2019, 25, 333. (in Chinese) |
| [49] | (曹丽娜, 陈炳辉, 苟习颖, 邹琦, 高等地质学报, 2019, 25, 333.) |
| [50] | Bi, S. F.; Cui, X. M. J. Salt. Chem. Ind. 2015, 44, 26. (in Chinese) |
| [50] | (毕思峰, 崔香梅, 盐业与化工, 2015, 44, 26.) |
/
| 〈 |
|
〉 |