

Copper Promoted Synthesis of Tetraalkylgermanes from Germanium Electrophiles and Alkyl Bromides※
Received date: 2021-12-30
Online published: 2022-03-17
Supported by
National Natural Science Foundation of China(22022109); National Natural Science Foundation of China(21871239)
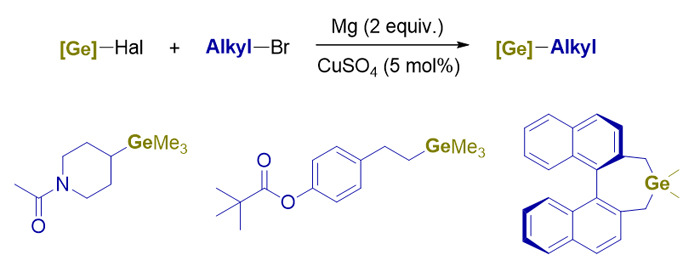
Organogermanium compounds have been gaining more attention for their unique properties compared to silicon or tin. Among which, tetraalkylgermanes, especially alkyltrimethylgermanes that have been confirmed to be active in photoredox radical reactions, are still lack of efficient and simple synthesis methods. Herein, we report a new protocol using commercially available trimethylgermanium bromide and alkyl bromides as substrates and cheap copper(II) sulfate as catalyst. When using magnesium powder as the reductant, a series of alkyltrimethylgermanes could be generated in moderate to good yield. Mechanism studies suggested a probable in-situ Grignard reaction pathway. The copper salt added could significantly accelerate the reaction between organohalogermanes and Grignard reagents so that the formation of Ge-Ge byproduct from the reduction of organohalogermanes by magnesium could be inhibited. Compared to the traditional method using Grignard reagent and organohalogermanes, this new protocol has better compatibility towards functional groups like esters and amides. The protocol could also be expanded to the synthesis of various tetraalkylgermanes or germacycloalkanes using dichlorodimethylgermane and alkyl bromides. General procedure for the synthesis of alkyltrimethylgermane is: To an oven-dried 25 mL screw-capped tube equipped with a stir bar was charge with 48 mg (2 mmol) magnesium powder and 8.0 mg (0.05 mmol) CuSO4. The tube was vacuumed and backfilled with argon for three cycles. 6 mL freshly distilled THF was added followed by the addition of 128 μL (1 mmol) trimethylgermanium bromide and 1.5 mmol alkyl bromide. The mixture was sealed with a Teflon stopper, warmed to 60 ℃ and stirred for 10 h. After cooled to room temperature, the resulted mixture was quenched with saturated NH4Cl solution, extracted with diethyl ether and washed with brine. Combined organic layer was dried over Na2SO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel or distillation to give the desired alkyltrimethylgermane.
Qinghao Xu , Lipu Wei , Zhen Zhang , Bin Xiao . Copper Promoted Synthesis of Tetraalkylgermanes from Germanium Electrophiles and Alkyl Bromides※[J]. Acta Chimica Sinica, 2022 , 80(4) : 428 -431 . DOI: 10.6023/A21120608
| [1] | Glockling, F. Gmelin Handbook of Inorganic Chemistry Ge Organogermanium Compounds Part 2: Ge(CH3)3R and Ge(C2H5)3R Compounds, Springer-Verlag, Berlin, Heidelberg, 1988, pp. 1-295. |
| [2] | Xue, W.; Mao, W.; Zhang, L.; Oestreich, M. Angew. Chem. Int. Ed. 2019, 58, 6440. |
| [3] | (a) Keess, S.; Oestreich, M. Org. Lett. 2017, 19, 1898. |
| [3] | (b) Meißner, G.; Kretschmar, K.; Braun, T.; Kemnitz, E. Angew. Chem. Int. Ed. 2017, 56, 16338. |
| [3] | (c) de la Vega-Hernández, K.; Romain, E.; Coffinet, A.; Bijouard, K.; Gontard, G.; Chemla, F.; Ferreira, F.; Jackowski, O.; Perez-Luna, A. J. Am. Chem. Soc. 2018, 140, 17632. |
| [3] | (d) Debrauwer, V.; Turlik, A.; Rummler, L.; Prescimone, A.; Blanchard, N.; Houk, K. N.; Bizet, V. J. Am. Chem. Soc. 2020, 142, 11153. |
| [3] | (e) Queen, A. E.; Selmani, A.; Schoenebeck, F. Org. Lett. 2022, 24, 406. |
| [4] | Su, P. F.; Wang, K.; Peng, X.; Pang, X.; Guo, P.; Shu, X. Z. Angew. Chem. Int. Ed. 2021, 60, 26571. |
| [5] | For Ni catalyzed reductive coupling reactions, see: (a) Gua, J.; Wan, g, X.; Xue, W.; Gong, H. Org. Chem. Front. 2015, 2, 1411. |
| [5] | (b) Poremba, K. E.; Dibrell, S. E.; Reisman, S. E. ACS Catal. 2020, 10, 8237. |
| [5] | (c) Zhang, L.; Oestreich, M. Angew. Chem. Int. Ed. 2021, 60, 18587. |
| [5] | (d) Duan, J.; Wang, K.; Xu, G.-L.; Kang, S.; Qi, L.; Liu, X.-Y.; Shu, X.-Z. Angew. Chem. Int. Ed. 2020, 59, 23083. |
| [5] | (e) Cheng, L.; Zhou, Q.-L. Acta Chim. Sinica 2020, 78, 1017. (in Chinese) |
| [5] | (程磊, 周其林, 化学学报, 2020, 78, 1017.) |
| [5] | (f) Li, Y.-Q.; Fan, Y.-H.; Jia, Q.-F. Chin. J. Org. Chem. 2019, 39, 350. (in Chinese) |
| [5] | (李娅琼, 范玉航, 贾乾发, 有机化学, 2019, 39, 350.) |
| [6] | During this manuscript is under revision, Shu group reported a nickel catalyzed reductive coupling reaction between Me3GeCl and alkyl bromide using Mn as reductant, see: Guo, P.; Pang, X.; Wang, K.; Su, P.-F.; Pan, Q.-Q.; Han, G.-Y.; Shen, Q.; Zhao, Z.-Z.; Zhang, W.; Shu, X.-Z. Org. Lett. 2022, 24, 1802. |
| [7] | Xu, Q. H.; Wei, L. P.; Xiao, B. Angew. Chem. Int. Ed. 2022, DOI: 10.1002/anie.202115592. |
| [8] | Eiji, M.; Kei, M.; Masayuki, I.; Koji, H.; Hideki, Y.; Koichiro, O. Bull. Chem. Soc. Jpn. 2009, 82, 1012. |
| [9] | For Cu catalyzed reductive coupling reaction using Mg as reductant, see: (a) Liu, J.-H.; Yang, C.-T.; Lu, X.-Y.; Zhang, Z.-Q.; Xu, L.; Cui, M.; Lu, X.; Xiao, B.; Fu, Y.; Liu, L.. Chem. Eur. J. 2014, 20, 15334. |
| [9] | (b) Maaliki, C.; Thiery, E.; Thibonnet, J. Eur. J. Org. Chem. 2017, 2017, 209. |
| [9] | (c) Cheng, L.-J.; Mankad, N. P. Chem. Soc. Rev. 2020, 49, 8036. |
| [9] | (d) Han, B.-S.; Shi, Z.; He, H.-H.; Zhang, X.-H. Chin. J. Org. Chem. 2021, 41, 695. (in Chinese) |
| [9] | (韩博士, 时郑, 何慧红, 张兴华, 有机化学, 2021, 41, 695.) |
| [10] | Yields of the reaction between Et3GeCl and CyBr under the condition with/without CuSO4 are 90% and 84% respectively. The result may be attributed to the relative inertness of Et3GeCl towards reductive dimerization. Considering that alkyl-GeEt3 as radical precursor would lead to the competition between ethyl radical and the target alkyl radical, this manuscript mainly focus on the synthesis of alkyl-GeMe3. |
| [11] | Bhatt, S.; Nayak, S. K. Tetrahedron Lett. 2006, 47, 8395. |
| [12] | We thus checked GC-MS result of the reactions in Table 2 and Me6Ge2 less than 5% was found for almost all substrates. |
| [13] | (a) Barrau, J.; Rima, G.; Amine, M. E.; Satgé, J. Synth. React. Inorg. Met.-Org. Chem. 1988, 18, 21. |
| [13] | (b) Hölbling, M.; Masters, S. L.; Flock, M.; Baumgartner, J.; Hassler, K.; Robertson, H. E.; Wann, D. A. Inorg. Chem. 2008, 47, 3023. |
| [13] | (c) Yoder, C. H.; Crouse, J. E.; Wilson, D. A. J. Organomet. Chem. 1984, 276, 9. |
/
| 〈 |
|
〉 |