

Synthesis and Properties of Novel Circularly Polarized Luminescence Materials Based on Binaphthol Skeleton
Received date: 2022-03-19
Online published: 2022-04-25
Supported by
National Natural Science Foundation of China(21772012)
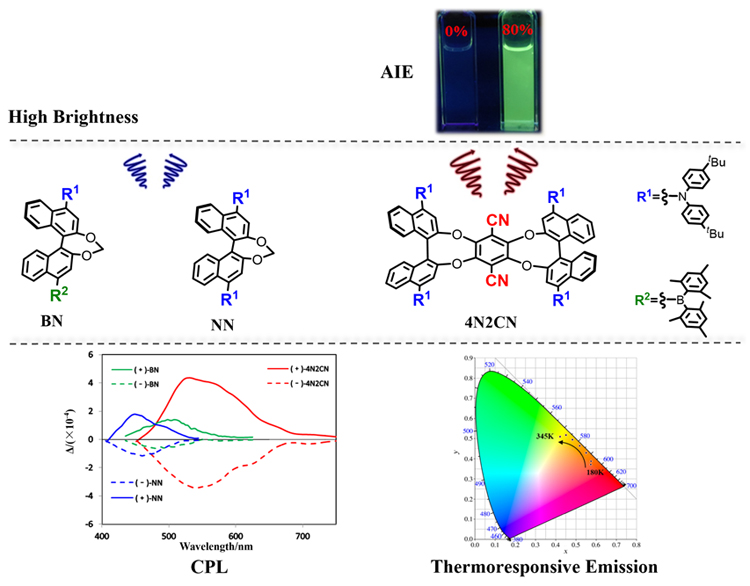
A series of chiral luminescent molecules based on binaphthol skeleton and having electron donor diarylamine (NN), electron donor/acceptor diarylamine and diarylboron (BN), electron donor/acceptor diarylamine and cyano (4N2CN) were synthesized. The properties of several molecules were compared by introducing different acceptor groups and extended molecular chiral skeletons. Compounds BN and 4N2CN exhibit significant thermochromic shift of the emission due to the intramolecular charge transfer (ICT) character. The emission wavelength was linearly enhanced with a high correlation coefficient of 0.991 for BN between 345 K and 150 K, 0.996 for 4N2CN between 345 K and 180 K. Furthermore, a fully reversible emission response was corroborated by an excellent fatigue resistance without emission degradation upon its exposure to 5 cycles of temperature change over a range of 195 K and 165 K for BN and 4N2CN. Optical resolution of the racemic mixtures have been performed for further investigation. The initial injection of these molecules into a chiral high performance liquid chromatography (HPLC) with a Daicel Chiralpak IA column resulted in two separated peaks with 1∶1 area. We try to further explore their chiral optical properties, including circular dichroism (CD) and circular polarized luminescence (CPL). The signal of CD indicates their chiral properties in the ground state. However, the difference of CPL signals of these compounds shows that the difference of group and skeleton will affect the chiral properties of molecules in the excited state. BN showed high brightness and ΦL of BN in solution can reach 0.99. In addition, 4N2CN exhibit aggregation-induced emission (AIE) properties in polar solvent and dynamic light scattering (DLS) was employed to monitor the process of aggregate formation. Based on this, we believe that the skeleton expansion and the introduction of electron-donor/acceptor groups make 4N2CN have unique photophysical properties. This work not only expands the family of chiral optical structures but also provides an idea to enhance the CPL signal and glum values.
Bin Liu , Pangkuan Chen . Synthesis and Properties of Novel Circularly Polarized Luminescence Materials Based on Binaphthol Skeleton[J]. Acta Chimica Sinica, 2022 , 80(7) : 929 -935 . DOI: 10.6023/A22030122
| [1] | (a) Mandal, P. K.; Collie, G. W.; Kauffmann, B.; Huc, I. Angew. Chem., Int. Ed. 2014, 53, 14424. |
| [1] | (b) Xiao, W.; Ernst, K. H.; Palotas, K.; Zhang, Y.; Bruyer, E.; Peng, L.; Greber, T.; Hofer, W. A.; Scott, L. T.; Fasel, R. Nat. Chem. 2016, 8, 326. |
| [1] | (c) Gonźalez-Rubio, G.; Liz-Marzán, L. M. Nature 2018, 556, 313. |
| [2] | (a) Nakamura, K.; Furumi, S.; Takeuchi, M.; Shibuya, T.; Tanaka, K. J. Am. Chem. Soc. 2014, 136, 5555. |
| [2] | (b) Cruz, C. M.; CastroFernández, S.; Mac?ôas, E.; Cuerva, J. M.; Campan?a, A. G. Angew. Chem., Int. Ed. 2018, 57, 14782. |
| [2] | (c) Han, X.-N.; Han, Y.; Chen, C.-F. J. Am. Chem. Soc. 2020, 142, 8262. |
| [2] | (d) Li, M.; Lin, W.-B.; Fang, L.; Chen, C.-F. Acta Chim. Sinica 2017, 75, 1150. (in Chinese) |
| [2] | (李猛, 林伟彬, 房蕾, 陈传峰, 化学学报, 2017, 75, 1150.) |
| [2] | (e) Song, L.-F.; Zhou, Y.-Y.; Gao, T.; Yan, P.-F.; Li, H.-F. Acta Chim. Sinica 2021, 79, 1042. (in Chinese) |
| [2] | (宋龙飞, 周妍妍, 高婷, 闫鹏飞, 李洪峰, 化学学报, 2021, 79, 1042.) |
| [3] | (a) Carr, R.; Evans, N. H.; Parker, D. Chem. Soc. Rev. 2012, 41, 7673. |
| [3] | (b) Takaishi, K.; Yasui, M.; Ema, T. J. Am. Chem. Soc. 2018, 140, 5334. |
| [3] | (c) Ma, J.-L.; Peng, Q.; Zhao, C.-H. Chem. - Eur. J. 2019, 25, 15441. |
| [4] | (a) Grell, M.; Oda, M.; Whitehead, K. S.; Asimakis, A.; Neher, D.; Bradley, D. D. C. Adv. Mater. 2001, 13, 577. |
| [4] | (b) Li, M.; Li, S.-H.; Zhang, D.; Cai, M.; Duan, L.; Fung, M.-K.; Chen, C.-F. Angew. Chem., Int. Ed. 2018, 57, 2889. |
| [4] | (c) Zinna, F.; Voci, S.; Arrico, L.; Brun, E.; Homberg, A.; Bouffier, L.; Funaioli, T.; Lacour, J.; Sojic, N.; Di Bari, L. Angew. Chem., Int. Ed. 2019, 58, 6952. |
| [4] | (d) Liang, Z.-P.; Tang, R.; Qiu, Y.-C.; Wang, Y.; Lu, H.-B.; Wu, Z.-G. Acta Chim. Sinica 2021, 79, 1401. (in Chinese) |
| [4] | (梁志鹏, 唐瑞, 邱雨晨, 王阳, 陆洪彬, 吴正光, 化学学报, 2021, 79, 1401.) |
| [5] | (a) Zinna, F.; Giovanella, U.; Bari, L. D. Adv. Mater. 2015, 27, 1791. |
| [5] | (b) Brandt, J. R.; Salerno, F.; Fuchter, M. J. Nat. Rev. Chem. 2017, 1, 0045. |
| [5] | (c) Song, F.; Xu, Z.; Zhang, Q.; Zhao, Z.; Zhang, H.; Zhao, W.; Qiu, Z.; Qi, C.; Zhang, H.; Sung, H. H. Y.; Williams, I. D.; Lam, J. W. Y.; Zhao, Z.; Qin, A.; Ma, D.; Tang, B. Z. Adv. Funct. Mater. 2018, 28, 1800051. |
| [6] | (a) Tang, Y.; Cohen, A. E. Science 2011, 332, 333. |
| [6] | (b) Takaishi, K.; Yamamoto, T.; Hinoide, S.; Ema, T. Chem. - Eur. J. 2017, 23, 9249. |
| [6] | (c) He, C.; Yang, G.; Kuai, Y.; Shan, S.; Yang, L.; Hu, J.; Zhang, D.; Zhang, Q.; Zou, G. Nat. Commun. 2018, 9, 5117. |
| [7] | (a) Takaishi, K.; Hinoide, S.; Matsumoto, T.; Ema, T. J. Am. Chem. Soc. 2019, 141, 11852. |
| [7] | (b) Jiang, Q.; Xu, X.; Yin, P.-A.; Ma, K.; Zhen, Y.; Duan, P.; Peng, Q.; Chen, W.-Q.; Ding, B. J. Am. Chem. Soc. 2019, 141, 9490. |
| [7] | (c) Zhang, C.; Yan, Z.-P.; Dong, X.-Y.; Han, Z.; Li, S.; Fu, T.; Zhu, Y.-Y.; Zheng, Y.-X.; Niu, Y.-Y.; Zang, S.-Q. Adv. Mater. 2020, 32, 2002914. |
| [8] | (a) Han, J.; Duan, P.; Li, X.; Liu, M. J. Am. Chem. Soc. 2017, 139, 9783. |
| [8] | (b) Liang, X.; Liu, T.-T.; Yan, Z.-P.; Zhou, Y.; Su, J.; Luo, X.-F.; Wu, Z.-G.; Wang, Y.; Zheng, Y.-X.; Zuo, J.-L. Angew. Chem., Int. Ed. 2019, 58, 17220. |
| [8] | (c) Zhao, W.-L.; Li, M.; Lu, H.-Y.; Chen, C.-F. Chem. Commun. 2019, 55, 13793. |
| [9] | (a) Sanchez-Carnerero, E. M.; Moreno, F.; Maroto, B. L.; Agarrabeitia, A. R.; Ortiz, M. J.; Vo, B. G.; Muller, G.; de la Moya, S. J. Am. Chem. Soc. 2014, 136, 3346. |
| [9] | (b) Kumar, J.; Tsumatori, H.; Yuasa, J.; Kawai, T.; Nakashima, T. Angew. Chem., Int. Ed. 2015, 54, 5943. |
| [9] | (c) Takaishi, K.; Yamamoto, T.; Hinoide, S.; Ema, T. Chem. - Eur. J. 2017, 23, 9249. |
| [10] | (a) Field, J. E.; Muller, G.; Riehl, J. P.; Venkataraman, D. J. Am. Chem. Soc. 2003, 125, 11808. |
| [10] | (b) Sawada, Y.; Furumi, S.; Takai, A.; Takeuchi, M.; Noguchi, K.; Tanaka, K. J. Am. Chem. Soc. 2012, 134, 4080. |
| [10] | (c) Katayama, T.; Nakatsuka, S.; Hirai, H.; Yasuda, N.; Kumar, J.; Kawai, T.; Hatakeyama, T. J. Am. Chem. Soc. 2016, 138, 5210. |
| [10] | (d) Otani, T.; Tsuyuki, A.; Iwachi, T.; Someya, S.; Tateno, K.; Kawai, H.; Saito, T.; Kanyiva, K. S.; Shibata, T. Angew. Chem., Int. Ed. 2017, 56, 3906. |
| [10] | (e) Qiu, Z.; Ju, C.-W.; Frédéric, L.; Hu, Y.; Schollmeyer, D.; Pieters, G.; Mu?llen, K.; Narita, A. J. Am. Chem. Soc. 2021, 143, 4661. |
| [11] | (a) Morisaki, Y.; Gon, M.; Sasamori, T.; Tokitoh, N.; Chujo, Y. J. Am. Chem. Soc. 2014, 136, 3350. |
| [11] | (b) Gon, M.; Morisaki, Y.; Chujo, Y. J. Mater. Chem. C 2015, 3, 521. |
| [11] | (c) Chen, J.-F.; Yin, X.; Wang, B.; Zhang, K.; Meng, G.; Zhang, S.; Shi, Y.; Wang, N.; Wang, S.; Chen, P. Angew. Chem., Int. Ed. 2020, 59, 11267. |
| [12] | (a) Lee, S.; Kaib, P. S. J.; List, B. J. Am. Chem. Soc. 2017, 139, 2156. |
| [12] | (b) Takaishi, K.; Hinoide, S.; Matsumoto, T.; Ema, T. J. Am. Chem. Soc. 2019, 141, 11852. |
| [12] | (c) Takaishi, K.; Iwachido, K.; Takehana, R.; Uchiyama, M.; Ema, T. J. Am. Chem. Soc. 2019, 141, 6185. |
| [13] | Kimoto, T.; Tajima, N.; Fujiki, M.; Imai, Y. Chem. Asian J. 2012, 7, 2836. |
| [14] | (a) Tsumatori, H.; Nakashima, T.; Kawai, T. Org. Lett. 2010, 12, 2362. |
| [14] | (b) Zhang, S.; Wang, Y.; Meng, F.; Dai, C.; Cheng, Y.; Zhu, C. Chem. Commun. 2015, 51, 9014. |
| [14] | (c) Zhang, K.; Zhao, J. Y.; Zhang, N.; Chen, J. F.; Wang, N.; Yin, X. D.; Zheng, X. Y.; Chen, P. K. J. Mater. Chem. C 2022, 10, 1816. |
| [15] | (a) Entwistle, C. D.; Marder, T. B. Angew. Chem., Int. Ed. 2002, 41, 2927. |
| [15] | (b) Zhou, G.; Ho, C.-L.; Wong, W.-Y.; Wang, Q.; Ma, D.; Wang, L.; Lin, Z.; Marder, T. B.; Beeby, A. Adv. Funct. Mater. 2008, 18, 499. |
| [15] | (c) Sun, Z.-B.; Liu, J.-K.; Yuan, D.-F.; Zhao, Z.-H.; Zhu, X.-Z.; Liu, D.-H.; Peng, Q.; Zhao, C.-H. Angew. Chem., Int. Ed. 2019, 58, 4840. |
| [15] | (d) Chen, J.-F.; Yin, X.; Zhang, K.; Zhao, Z.; Zhang, S.; Zhang, N.; Wang, N.; Chen, P. J. Org. Chem. 2021, 86, 12654. |
| [15] | (e) Xia, Z.-Q.; Shao, A.-D.; Li, Q.; Zhu, S.-Q.; Zhu, W.-H. Acta Chim. Sinica 2016, 74, 351. (in Chinese) |
| [15] | (夏志清, 邵安东, 李强, 朱世琴, 朱为宏, 化学学报, 2016, 74, 351.) |
| [15] | (f) Wang, T.; Hua, X.; Yu, Y.; Yuan, Y.; Feng, M.; Jiang, Z. Chin. J. Org. Chem. 2019, 39, 1436. (in Chinese) |
| [15] | (王彤彤, 华晓晨, 郁友军, 袁熠, 冯敏强, 蒋佐权, 有机化学, 2019, 39, 1436.) |
| [15] | (g) Yu, J.; Xiao, Y.; Chen, J. Chin. J. Org. Chem. 2019, 39, 3460. (in Chinese) |
| [15] | (俞佳, 肖雅方, 陈嘉雄, 有机化学, 2019, 39, 3460.) |
| [16] | (a) Zhao, W.; Zhuang, X.; Wu, D.; Zhang, F.; Gehrig, D.; Laquai, F.; Feng, X. J. Mater. Chem. A 2013, 1, 13878. |
| [16] | (b) Sudhakar, P.; Mukherjee, S.; Thilagar, P. Organometallics 2013, 32, 3129. |
| [17] | (a) Liu, Z.-Q.; Shi, M.; Li, F.-Y.; Fang, Q.; Chen, Z.-H.; Yi, T.; Huang, C.-H. Org. Lett. 2005, 7, 5481. |
| [17] | (b) Stahl, R.; Lambert, C.; Kaiser, C.; Wortmann, R.; Jakober, R. Chem. - Eur. J. 2006, 12, 2358. |
| [18] | (a) Yuan, Z.; Entwistle, C. D.; Collings, J. C.; Albesa-Jové, D.; Batsanov, A. S.; Howard, J. A. K.; Taylor, N. J.; Kaiser, H. M.; Kaufmann, D. E.; Poon, S.-Y.; Wong, W.-Y.; Jardin, C.; Fathallah, S.; Boucekkine, A.; Halet, J.-F.; Marder, T. B. Chem. - Eur. J. 2006, 12, 2758. |
| [18] | (b) Cao, D.; Liu, Z.; Fang, Q.; Xu, G.; Xue, G.; Liu, G.; Yu, W. J. Organomet. Chem. 2004, 689, 2201. |
| [18] | (c) Liu, Z.; Fang, Q.; Wang, D.; Cao, D.; Xue, G.; Yu, W.; Lei, H. Chem. - Eur. J. 2003, 9, 5074. |
| [19] | (a) Shirota, Y.; Kinoshita, M.; Noda, T.; Okumoto, K.; Ohara, T. J. Am. Chem. Soc. 2000, 122, 11021. |
| [19] | (b) Doi, H.; Kinoshita, M.; Okumoto, K.; Shirota, Y. Chem. Mater. 2003, 15, 1080. |
| [19] | (c) Hudson, Z. M.; Sun, C.; Helander, M. G.; Amarne, H.; Lu, Z. H.; Wang, S. Adv. Funct. Mater. 2010, 20, 3426. |
| [20] | (a) Duan, L.; Qiao, J.; Sun, Y.; Qiu, Y. Adv. Mater. 2011, 23, 1137. |
| [20] | (b) Zhang, D.; Song, X.; Gillett, A. J.; Drummond, B. H.; Jones, S. T. E.; Li, G.; He, H.; Cai, M.; Credgington, D.; Duan, L. Adv. Mater. 2020, 32, 1908355. |
| [21] | MacLean, M. W.; Wood, T. K.; Wu, G.; Lemieux, R. P.; Crudden, C. M. Chem. Mater. 2014, 26, 5852. |
| [22] | Zang, E.-S.; Hou, X.-F.; Zang, Z.; Zhang, Y.-Q.; Wang, J.-J.; Yang, H.; You, J.-M.; Ju, P. J. Mater. Chem. C 2019, 7, 8404. |
| [23] | (a) Hong, Y.; Lam, J. W. Y.; Tang, B. Z. Chem. Soc. Rev. 2011, 40, 5361. |
| [23] | (b) Zhang, Y.; Li, D.; Li, Y.; Yu, J. Chem. Sci. 2014, 5, 2710. |
| [23] | (c) Gu, Y.; Zhao, Z.; Su, H.; Zhang, P.; Liu, J.; Niu, G.; Li, S.; Wang, Z.; Kwok, R. T. K.; Ni, X.-L.; Sun, J.; Qin, A.; Lam, J. W. Y.; Tang, B. Z. Chem. Sci. 2018, 9, 6497. |
| [23] | (d) Zhao, Z.; Zhang, H.; Lam, J. W. Y.; Tang, B. Z. Angew. Chem., Int. Ed. 2020, 59, 9888. |
| [24] | Zhang, Z.; Edkins, R. M.; Nitsch, J.; Fucke, K.; Steffen, A.; Longobardi, L. E.; Stephan, D. W.; Lambert, C.; Marder, T. B. Chem. Sci. 2015, 6, 308. |
/
| 〈 |
|
〉 |