

W-doped Hierarchically Porous Silica Nanosphere Supported Platinum for Catalytic Glycerol Hydrogenolysis to 1,3-Propanediol
Received date: 2022-02-01
Online published: 2022-05-24
Supported by
National Key Research and Development Project of China(2016YFB0301602); State Key Laboratory of Catalytic Materials and Reaction Engineering (RIPP, SINOPEC); National Natural Science Foundation of China(21872035); Science and Technology Commission of Shanghai Municipality(19DZ2270100)
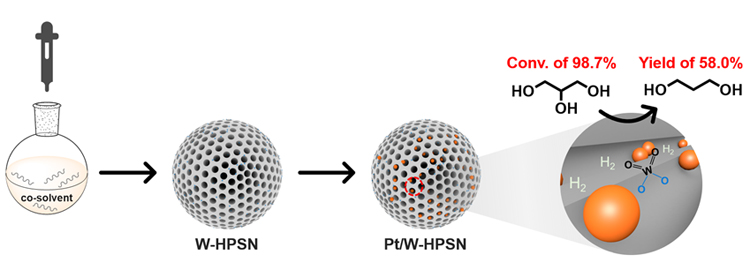
As a versatile platform molecule, glycerol has been widely studied for the production of high value-added chemicals. In particular, catalytic hydrogenolysis of glycerol to 1,3-propanediol (1,3-PDO) is a highly desired route for glycerol valorization. Herein, hierarchically porous SiO2 nanospheres doped in situ with W (W-HPSN) were synthesized. The effect of the addition of short-chain alcohols (methanol, ethanol, and n-propanol) as co-solvents during the synthesis of W-HPSN on the catalytic performances of the Pt/W-HPSN catalysts in glycerol hydrogenolysis to 1,3-PDO was systematically investigated. The basic physicochemical properties, the chemical states of the active components, and the acidic properties of the catalysts were characterized by a variety of techniques. Compared with the Pt/W-HPSN-H2O catalyst prepared from W-HPSN synthesized only with water as the solvent, when the alcohols were added as the co-solvents, the specific surface area of the catalyst increased to different degrees. And aside from the micropores at 1.4 nm and the mesopores at >2 nm, new micropores appeared at 1.7 nm. In glycerol hydrogenolysis, the catalysts prepared from the W-HPSN synthesized with the addition of alcohols as the co-solvents also displayed improved glycerol conversion and 1,3-PDO selectivity, and the 1,3-PDO yields were in the order of Pt/W-HPSN-Me>Pt/W-HPSN-Pr>Pt/W-HPSN-Et>Pt/W-HPSN-H2O. On the best Pt/W-HPSN-Me catalyst synthesized with methanol as the co-solvent, the glycerol conversion and 1,3-PDO selectivity were 88.8% and 56.3%, respectively, in comparison to 64.1% and 40.7%, respectively, on the Pt/W-HPSN-H2O catalyst. Elemental analysis showed that the Pt and W loadings on the Pt/W-HPSN-H2O and Pt/W-HPSN-Me catalysts are identical. The X-ray photoelectron spectroscopy (XPS), Raman, and ultraviolet-visible diffuse reflectance spectroscopy (UV-Vis DRS) characteri-zations revealed that the chemical states of the Pt and W species on the Pt/W-HPSN-H2O and Pt/W-HPSN-Me catalysts are similar. CO chemisorption and transmission electron microscopy (TEM) characterizations demonstrated that the Pt particle size on the Pt/W-HPSN-Me catalyst is smaller than that on the Pt/W-HPSN-H2O catalyst. And the cumene cracking reaction detected more in-situ generated Brønsted acid sites on the Pt/W-HPSN-Me catalyst than on the Pt/W-HPSN-H2O catalyst in H2 atmosphere. On the basis of these characterization results, we propose that smaller Pt particle size and more in-situ generated Brønsted acid sites are conducive to a better catalytic performance of the Pt/W-HPSN catalyst. By further optimization of the composition of the W-HPSN-Me support, at the W/Si molar ratio of 1/320 and under the reaction conditions of 423 K, 4 MPa of H2 pressure, and reaction time of only 12 h, the Pt/W-HPSN-Me catalyst afforded enhanced glycerol conversion and 1,3-PDO selectivity of 98.7% and 58.8%, respectively, thus giving rise to an outstanding 1,3-PDO yield of 58.0%. This work shows prospect for the HPSN material as an excellent catalyst support for the hydrogenolysis of glycerol to 1,3-PDO.
Yang Zeng , Lan Jiang , Xiaoxin Zhang , Songhai Xie , Yan Pei , Minghua Qiao , Zhen-Hua Li , Hualong Xu , Kangnian Fan , Baoning Zong . W-doped Hierarchically Porous Silica Nanosphere Supported Platinum for Catalytic Glycerol Hydrogenolysis to 1,3-Propanediol[J]. Acta Chimica Sinica, 2022 , 80(7) : 903 -912 . DOI: 10.6023/A22020059
| [1] | Ragauskas, A. J.; Williams, C. K.; Davison, B. H.; Britovsek, G.; Cairney, J.; Eckert, C. A.; Frederick, W. J.; Hallett, J. P.; Leak, D. J.; Liotta, C. L.; Mielenz, J. R.; Murphy, R.; Templer, R.; Tschaplinski, T. Science 2006, 311, 484. |
| [2] | Besson, M.; Gallezot, P.; Pinel, C. Chem. Rev. 2014, 114, 1827. |
| [3] | Nanda, M. R.; Yuan, Z. S.; Qin, W. S.; Ghaziaskar, H. S.; Poirier, M. A.; Xu, C. B. Fuel 2014, 117, 470. |
| [4] | Zhou, C. H.; Zhao, H.; Tong, D. S.; Wu, L. M.; Yu, W. H. Catal. Rev. 2013, 55, 369. |
| [5] | Tan, H. W.; Aziz, A. A.; Aroua, M. K. Renewable Sustainable Energy Rev. 2013, 27, 118. |
| [6] | Zhang, G. L.; Ma, B. B.; Xu, X. L.; Li, C.; Wang, L. W. Biochem. Eng. J. 2007, 37, 256. |
| [7] | Saxena, R. K.; Anand, P.; Saran, S.; Isar, J. Biotechnol. Adv. 2009, 27, 895. |
| [8] | Min, E. Z.; Wu, W. Green Chemistry and Engineering, Chemical Industry Press, Beijing, 2001, pp. 54-61. (in Chinese) |
| [8] | (闵恩泽, 吴巍, 绿色化学与化工, 化学工业出版社, 北京, 2001, pp. 54-61.) |
| [9] | Yazdani, S. S.; Gonzalez, R. Curr. Opin. Biotechnol. 2007, 18, 213. |
| [10] | Qian, B. Z. Biomass Energy Technologies and Applications, Science Press, Beijing, 2010, pp. 204-206. (in Chinese) |
| [10] | (钱伯章, 生物质能技术与应用, 科学出版社, 北京, 2010, pp. 204-206.) |
| [11] | Urban, R. A.; Bakshi, B. R. Ind. Eng. Chem. Res. 2009, 48, 8068. |
| [12] | Numpilai, T.; Cheng, C. K.; Seubsai, A.; Faungnawakij, K.; Limtrakul, J.; Witoon, T. Environ. Pollut. 2021, 272, 116029. |
| [13] | Wu, F. L.; Jiang, H. F.; Zhu, X. H.; Lu, R.; Shi, L.; Lu, F. ChemSusChem 2021, 14, 569. |
| [14] | Zhao, D. Y.; Feng, J. L.; Huo, Q. S.; Melosh, N.; Fredrickson, G. H.; Chmelka, B. F.; Stucky, G. D. Science 1998, 279, 548. |
| [15] | Davis, M. E. Nature 2002, 417, 813. |
| [16] | Feng, Q. C.; Zhao, S.; Xu, Q.; Chen, W. X.; Tian, S. B.; Wang, Y.; Yan, W. S.; Wang, D. S.; Luo, J.; Li, Y. D. Adv. Mater. 2019, 31, 1901024. |
| [17] | Gu, M. Y.; Shen, Z.; Yang, L.; Peng, B. Y.; Dong, W. J.; Zhang, W.; Zhang, Y. L. Ind. Eng. Chem. Res. 2017, 56, 13572. |
| [18] | Priya, S. S.; Kumar, V. P.; Kantam, M. L.; Bhargava, S. K.; Srikanth, A.; Chary, K. V. Ind. Eng. Chem. Res. 2015, 54, 9104. |
| [19] | Feng, S. H.; Zhao, B. B.; Liang, Y.; Liu, L.; Dong, J. X. Ind. Eng. Chem. Res. 2019, 58, 2661. |
| [20] | Fan, Y. Q.; Cheng, S. J.; Wang, H.; Ye, D. H.; Xie, S. H.; Pei, Y.; Hu, H. R.; Hua, W. M.; Li, Z. H.; Qiao, M. H.; Zong, B. N. Green Chem. 2017, 19, 2174. |
| [21] | Cheng, S. J.; Zeng, Y.; Pei, Y.; Fan, K. N.; Qiao, M. H.; Zong, B. N. Acta Chim. Sinica 2019, 77, 1054. (in Chinese) |
| [21] | (成诗婕, 曾杨, 裴燕, 范康年, 乔明华, 宗保宁, 化学学报, 2019, 77, 1054.) |
| [22] | Yang, P. D.; Deng, T.; Zhao, D. Y.; Feng, P. Y.; Pine, D.; Chmelka, B. F.; Whitesides, G. M.; Stucky, G. D. Science 1998, 282, 2244. |
| [23] | Gao, X.; Pan, H. B.; He, Z. X.; Yang, K.; Qiao, C. F.; Liu, Y. L.; Zhou, C. S. Acta Chim. Sinica 2021, 79, 1502. (in Chinese) |
| [23] | (高霞, 潘会宾, 贺曾贤, 杨柯, 乔成芳, 刘永亮, 周春生, 化学学报, 2021, 79, 1502.) |
| [24] | Mu, C. H.; Zhang, Y. X.; Kou, W.; Xu, L. B. Acta Chim. Sinica 2021, 79, 925. (in Chinese) |
| [24] | (穆春辉, 张艺馨, 寇伟, 徐联宾, 化学学报, 2021, 79, 925.) |
| [25] | Zhao, Y. J.; Guo, Z. Y.; Zhang, H. J.; Peng, B.; Xu, Y. X.; Wang, Y.; Zhang, J.; Xu, Y.; Wang, S. P.; Ma, X. B. J. Catal. 2018, 357, 223. |
| [26] | Yue, D.; Lei, J. H.; Peng, Y.; Li, J. S.; Du, X. D. J. Porous Mater. 2018, 25, 727. |
| [27] | Matsuyama, K.; Tanaka, S.; Kato, T.; Okuyama, T.; Muto, H.; Miyamoto, R.; Bai, H. Z. J. Supercrit. Fluids 2017, 130, 140. |
| [28] | Wang, H.; Pinnavaia, T. J. Angew. Chem. Int. Ed. 2006, 118, 7765. |
| [29] | Wang, X. D.; Gao, X. X.; Dong, M.; Zhao, H. B.; Huang, W. J. Energy Chem. 2015, 24, 490. |
| [30] | Du, X.; He, J. H. Langmuir 2010, 26, 10057. |
| [31] | Shimizu, W.; Hokka, J.; Sato, T.; Usami, H.; Murakami, Y. J. Phys. Chem. B 2011, 115, 9369. |
| [32] | Liu, J. L.; Zhu, L. J.; Pei, Y.; Zhuang, J. H.; Li, H.; Li, H. X.; Qiao, M. H.; Fan, K. N. Appl. Catal. A 2009, 353, 282. |
| [33] | Cheng, S. J.; Fan, Y. Q.; Zhang, X. X.; Zeng, Y.; Xie, S. H.; Pei, Y.; Zeng, G. F.; Qiao, M. H.; Zong, B. N. Appl. Catal. B 2021, 297, 120428. |
| [34] | Nie, Y. Y.; Shang, S. N.; Xin, X.; Hua, W. M.; Yue, Y. H.; Gao, Z. Appl. Catal. A 2012, 433-434, 69. |
| [35] | Wu, K. H.; Zhou, Y. W.; Ma, X. Y.; Ding, C.; Cai, W. B. Acta Chim. Sinica 2018, 76, 292. (in Chinese) |
| [35] | (吴匡衡, 周亚威, 马宪印, 丁辰, 蔡文斌, 化学学报, 2018, 76, 292.) |
| [36] | Nagai, Y.; Hirabayashi, T.; Dohmae, K.; Takagi, N.; Minami, T.; Shinjoh, H.; Matsumoto, S. J. Catal. 2006, 242, 103. |
| [37] | Lewandowska, A. E.; Banares, M. A.; Tielens, F.; Che, M.; Dzwigaj, S. J. Phys. Chem. C 2010, 114, 19771. |
| [38] | Zhang, Z. Y.; Zhu, Q. J.; Ding, J.; Dai, W. L.; Zong, B. N. Acta Phys.-Chim. Sin. 2014, 30, 1527. (in Chinese) |
| [38] | (张召艳, 祝全敬, 丁靖, 戴维林, 宗保宁, 物理化学学报, 2014, 30, 1527.) |
| [39] | Rada, S.; Rada, M.; Culea, E. J. Alloys Compd. 2013, 552, 10. |
| [40] | Jambhrunkar, S.; Yu, M. H; Yang, J.; Zhang, J.; Shrotri, A.; Endo-Munoz, L.; Moreau, J.; Lu, G. Q.; Yu, C. Z. J. Am. Chem. Soc. 2013, 135, 8444. |
| [41] | Yang, X. L.; Dai, W. L.; Chen, H.; Xu, J. H.; Cao, Y.; Li, H. X.; Fan, K. N. Appl. Catal. A 2005, 283, 1. |
| [42] | Ghosh, S.; Acharyya, S. S.; Sasaki, T.; Bal, R. Green Chem. 2015, 17, 1867. |
| [43] | Zhu, S. H.; Gao, X. Q.; Zhu, Y. L.; Zhu, Y. F.; Xiang, X. M.; Hu, C. X.; Li, Y. W. Appl. Catal. B 2013, 140-141, 60. |
| [44] | Parry, E. P. J. Catal. 1963, 2, 371. |
| [45] | Galano, A.; Rodriguez-Gattorno, G.; Torres-García, E. Phys. Chem. Chem. Phys. 2008, 10, 4181. |
| [46] | Zhu, S. H.; Qiu, Y. N.; Zhu, Y. L.; Hao, S. L.; Zheng, H. Y.; Li, Y. W. Catal. Today 2013, 212, 120. |
| [47] | Woolery, G. L.; Kuehl, G. H.; Timken, H. C.; Chester, A. W.; Vartuli, J. C. Zeolites 1997, 19, 288. |
| [48] | Zhou, W.; Li, Y.; Wang, X. F.; Yao, D. W.; Wang, Y., Huang, S. Y.; Li, W.; Zhao, Y. J.; Wang, S. P.; Ma, X. B. J. Catal. 2020, 388, 154. |
| [49] | Zhu, S. H.; Gao, X. Q.; Zhu, Y. L.; Li, Y. W. J. Mol. Catal. A 2015, 398, 391. |
| [50] | Gong, L. F.; Yuan, L.; Ding, Y. J.; Lin, R. H.; Li, J. W.; Dong, W. D.; Tao, W.; Chen, W. M. Appl. Catal. A 2010, 390, 119. |
| [51] | Inagaki, S., Shinoda, S.; Kaneko, Y.; Takechi, K.; Komatsu, R.; Tsuboi, Y.; Yamazaki, H.; Kondo, J. N.; Kubota, Y. ACS Catal. 2013, 3, 74. |
| [52] | Miao, G.; Shi, L.; Zhou, Z. M.; Zhu, L. J.; Zhang, Y. F.; Zhao, X. P.; Luo, H.; Li, S. G.; Kong, L. Z.; Sun, Y. H. ACS Catal. 2020, 10, 15217. |
| [53] | Barré, T.; Arurault, L.; Sauvage, F. X. Spectrochim. Acta Part A 2005, 61, 551. |
| [54] | Zhu, S. H.; Zhu, Y. L.; Hao, S. L.; Chen, L. G.; Zhang, B.; Li, Y. W. Catal. Lett. 2012, 142, 267. |
| [55] | Chen, H. M.; He, J. H.; Tang, H. M.; Yan, C. X. Chem. Mater. 2008, 20, 5894. |
/
| 〈 |
|
〉 |