

The SMSI of Pt-TiO2 During the Crystalline Phase Transformation and Its Effect on CO Oxidation Performance
Received date: 2022-04-08
Online published: 2022-06-14
Supported by
National Natural Science Foundation of China(21878121); Shandong Provincial Natural Science Foundation(ZR202102230042)
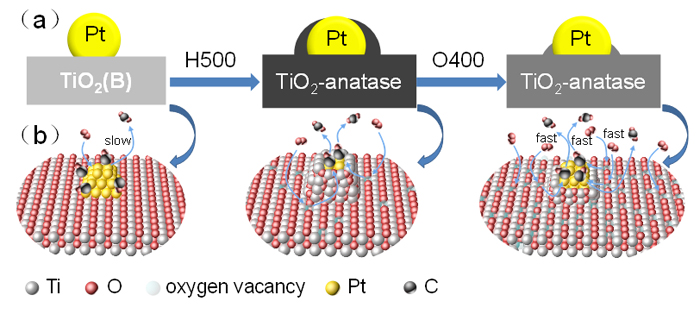
The strong metal-support interaction (SMSI) has long been studied in heterogonous catalysis on account of its importance in stabilizing active metals and tuning catalytic performance. So far, although there are many reports about SMSI in Pt-TiO2 system, they are mainly focusing on support structure sensitivity (crystal plane effect) and the size of supported noble metals (size effect), it is still unclear whether the TiO2 crystalline phase influences the creation of SMSI or not. In this study, we choose brookite TiO2 (TiO2(B)) as support to prepare Pt-TiO2(B) catalyst by means of traditional impregnation method. Then the as-prepared Ptimp/TiO2(B) was treated at different temperature under different atmosphere, specifically, 250 ℃ heat treatment under H2 (the sample is labeled as Ptimp/TiO2(B)-H250), 500 ℃ heat treatment under H2 (the sample is labeled as Ptimp/TiO2(B)-H500), and 400 ℃ heat treatment under O2 followed by H2 treatment at 500 ℃ (the sample is labeled as Ptimp/TiO2(B)-H500+O400). The experimental results show that the 500 ℃ heat treatment under H2 atmosphere induces the crystalline phase transformation of TiO2(B) into anatase, and more importantly, this transformation leads to the TiO2-x migration as a result of the encapsulation of Pt particles by TiO2-x. After further heat treatment at 400 ℃ under O2 atmosphere, the TiO2-x shell coated on the Pt nanoparticle surfaces was removed, which displays a classical SMSI behavior. According to the high resolution transmission electron microscope (HRTEM) and CO diffuse reflectance infrared Fourier transform spectroscopy (CO-DRIFTS) results, we proposed that the crystalline phase transformation plays a great role in the creation of SMSI. In addition, we selected CO oxidation as a model reaction to compare the catalytic activity of Ptimp/TiO2(B) catalysts. The results demonstrate that the activity of the catalysts is simultaneously affected by the TiO2-x coating on Pt nanoparticles and the oxygen vacancy concentration within the support. More specifically, compared with TiO2(B), anatase TiO2 has abundant oxygen vacancies, which is of great importance to activate O2. In the case of Ptimp/TiO2(B)-H500+O400 catalyst, the active sites of Pt nanoparticles are exposed due to the removal of TiO2-x coating, and the anatase TiO2 has a certain amount of oxygen vacancies. Therefore, Ptimp/TiO2(B)-H500+O400 catalyst exhibits the best activity among all compared Pt/TiO2(B) catalyst. Obviously, this work greatly enriches the SMSI for Pt/TiO2 system, and provides an important reference value for the creation of SMSI, if any, when other crystalline phase transformation (or phase transformation) involved supports are suitably chosen.
Yahui Jia , Chunsheng Li , Zhongzhen Xu , Wei Liu , Daowei Gao , Guozhu Chen . The SMSI of Pt-TiO2 During the Crystalline Phase Transformation and Its Effect on CO Oxidation Performance[J]. Acta Chimica Sinica, 2022 , 80(9) : 1289 -1298 . DOI: 10.6023/A22040162
| [1] | Tauster S. J.; Fung S. C. J. Catal. 1978, 55, 29. |
| [2] | Tang H. L.; Wei J. K.; Liu F.; Qiao B. T.; Pan X. L.; Li L.; Liu J. Y.; Wang J. H.; Zhang T. J. Am. Chem. Soc. 2016, 138, 56. |
| [3] | Tauster S. J.; Fung S. C.; Garten R. L. J. Am. Chem. Soc. 1978, 100, 170. |
| [4] | Figueiredo W. T.; Prakash R.; Vieira C. G.; Lima D. S.; Carvalho V. E.; Soares E. A.; Buchner S.; Raschke H.; Perez-Lopez O. W.; Baptista D. L.; Hergenroder R.; Segala M.; Bernardi F. Appl. Surf. Sci. 2022, 574, 151647. |
| [5] | Guo M.; Kong X. T.; Li C. Z.; Yang Q. H. Commun. Chem. 2021, 4, 54. |
| [6] | Shao J.-J.; Zhang P.; Song W.; Huang X.-M.; Xu Y.-D.; Shen W.-J. Acta Chim. Sinica 2007, 65, 2007.(in Chinese) |
| [6] | (邵建军, 张平, 宋巍, 黄秀敏, 徐奕德, 申文杰, 化学学报, 2007, 65, 2007.) |
| [7] | Fu Q.; Wagner T.; Olliges S.; Carstanjen H.-D. J. Phys. Chem. B 2005, 109, 944. |
| [8] | Zhang Y. S.; Liu J. X.; Qian K.; Jia A. P.; Li D.; Shi L.; Hu J.; Zhu J. F.; Huang W. X. Angew. Chem. Int. Ed. 2021, 60, 12074. |
| [9] | Ma D. Acta Phys.-Chim. Sin. 2022, 38, 9.(in Chinese) |
| [9] | (马丁, 物理化学学报, 2022, 38, 9.) |
| [10] | Neumann S.; Doebler H. H.; Keil S.; Erdt A. J.; Gutsche C.; Borchert H.; Kolny-Olesiak J.; Parisi J.; Baeumer M.; Kunz S. ACS Catal. 2020, 10, 4136. |
| [11] | Du X. R.; Huang Y. K.; Pan X. L.; Han B.; Su Y.; Jiang Q. K.; Li M. R.; Tang H. L.; Li G.; Qiao B. T. Nat. Commun. 2020, 11, 5811. |
| [12] | Chen H.; Yang Z. Z.; Wang X.; Polo-Garzon F.; Halstenberg P. W.; Wang T.; Suo X.; Yang S. Z.; Meyer H. M.; Wu Z. L.; Dai S. J. Am. Chem. Soc. 2021, 143, 8521. |
| [13] | Zhang J.; Zhu D. Z.; Yan J. F.; Wang C. A. Nat. Commun. 2021, 12, 6665. |
| [14] | Wang Y. L.; Zhang W.; Wang Z. H.; Cao Y. M.; Feng J. M.; Wang Z. L.; Ma Y. Chinese J. Catal. 2018, 39, 1500. |
| [15] | Choi H.; Lee J.; Kim D.; Kumar A.; Jeong B.; Kim K.-J.; Lee H.; Park J. Y. Catal. Sci. Technol. 2021, 11, 1698. |
| [16] | Li L.; Chen Y.; Jiao S. H.; Fang Z. X.; Liu X.; Xu Y.; Pang G. S.; Feng S. H. Mater. Design 2016, 100, 235. |
| [17] | Wang Z. H.; Wang Y. L.; Zhang W.; Wang Z. L.; Ma Y.; Zhou X. J. Phys. Chem. C 2019, 123, 1779. |
| [18] | Zhang J.; Xu Q.; Feng Z. C.; Li M. J.; Li C. Angew. Chem. Int. Ed. 2008, 47, 1766. |
| [19] | Liu J. H.; Ding T.; Zhang H.; Li G. C.; Cai J. M.; Zhao D. Y.; Tian Y.; Xian H.; Bai X. Q.; Li X. G. Catal. Sci. Technol. 2018, 8, 4934. |
| [20] | Chen Z. Y.; Liang L.; Yuan H.; Liu H.; Wu P.; Fu M. L.; Wu J. L.; Chen P. R.; Qiu Y. C.; Ye D. Q.; Chen L. M. Appl. Catal. B-Environ. 2021, 298, 120507. |
| [21] | D'Arienzo, M.; Carbajo, J.; Bahamonde, A.; Crippa, M.; Polizzi, S.; Scotti, R.; Wahba, L.; Morazzoni, F. J. Am. Chem. Soc. 2011, 133, 17652. |
| [22] | Xiao Q.; Wei S.; Wang W. W.; Jia C. J. Langmuir 2021, 37, 3270. |
| [23] | Guo X.-L.; Chen X.; Su D.-S.; Liang C.-H. Acta Chim. Sinica 2018, 76, 22.(in Chinese) |
| [23] | (郭小玲, 陈霄, 苏党生, 梁长海, 化学学报, 2018, 76, 22.) |
| [24] | Cai J. M.; Wu M. Q.; Wang Y. T.; Zhang H.; Meng M.; Tian Y.; Li X. G.; Zhang J.; Zheng L. R.; Gong J. L. Chem 2017, 2, 877. |
| [25] | Roberts S.; Gorte R. J. J. Catal. 1990, 124, 553. |
| [26] | Braunschweig E. J.; Logan A. D.; Datye A. K.; Smith D. J. J. Catal. 1989, 118, 227. |
| [27] | Xu D.; Wu B. S.; Ren P. J.; Wang S. Y.; Huo C. F.; Zhang B.; Guo W. P.; Huang L. H.; Wen X. D.; Qin Y.; Yang Y.; Li Y. W. Catal. Sci. Technol. 2017, 7, 1342. |
| [28] | Liu N.; Xu M.; Yang Y. S.; Zhang S. M.; Zhang J.; Wang W. L.; Zheng L. R.; Hong S.; Wei M. ACS Catal. 2019, 9, 2707. |
| [29] | Tang H. L.; Su Y.; Guo Y. L.; Zhang L. L.; Li T. B.; Zang K. T.; Liu F.; Li L.; Luo J.; Qiao B. T.; Wang J. H. Chem. Sci. 2018, 9, 6679. |
| [30] | DeRita L.; Dai S.; Lopez-Zepeda K.; Pham N.; Graham G. W.; Pan X.; Christopher P. J. Am. Chem. Soc. 2017, 139, 14150. |
| [30] | Liu S. F.; Qi H. F.; Zhou J. H.; Xu W.; Niu Y. M.; Zhang B. S.; Zhao Y.; Liu W.; Ao Z.; Kuang Z. C.; Li L.; Wang M.; Wang J. H. ACS Catal. 2021, 11, 6081. |
| [31] | Chen J.-M.; Cui C.-Q.; Liu H.-L.; Li G.-D. Acta Chim. Sinica 2022, 80, 467.(in Chinese) |
| [31] | (陈俊敏, 崔承前, 刘瀚林, 李国栋, 化学学报, 2022, 80, 467.) |
| [32] | Zhang X.-M.; Li X.-Y.; Xiong W.-F.; Li H.-F.; Cao R. Acta Chim. Sinica 2021, 79, 180.(in Chinese) |
| [32] | (张晓萌, 李希雅, 熊晚枫, 李红芳, 曹荣, 化学学报, 2021, 79, 180.) |
| [33] | Wu Q.-Y.; Qin R.-X.; Zang D.-D.; Zhang W.-Y.; Wu B.-H.; Zheng N.-F. Acta Chim. Sinica 2018, 76, 617.(in Chinese) |
| [33] | (吴庆远, 秦瑞轩, 臧丹丹, 张无用, 吴炳辉, 郑南峰, 化学学报, 2018, 76, 617.) |
| [34] | Cai J. M.; Wang Y. T.; Zhu Y. M.; Wu M. Q.; Zhang H.; Li X. G.; Jiang Z.; Meng M. ACS Appl. Mater. Inter. 2015, 7, 24987. |
| [35] | Jiang D.; Yao Y. G.; Li T. Y.; Wan G.; Pereira-Hernandez X. I.; Lu Y. B.; Tian J. S.; Khivantsev K.; Engelhard M. H.; Sun C. J.; Garcia-Vargas C. E.; Hoffman A. S.; Bare S. R.; Datye A. K.; Hu L. B.; Wang Y. Angew. Chem. Int. Ed. 2021, 60, 26054. |
| [36] | Chen G. Z.; Xu Q. H.; Yang Y.; Li C. C.; Huang T. Z.; Sun G. X.; Zhang S. X.; Ma D. L.; Li X. ACS Appl. Mater. Inter. 2015, 7, 23538. |
| [37] | Han B.; Guo Y. L.; Huang Y. K.; Xi W.; Xu J.; Luo J.; Qi H. F.; Ren Y. J.; Liu X. Y.; Qiao B. T.; Zhang T. Angew. Chem. Int. Ed. 2020, 59, 11824. |
| [38] | Liu P. X.; Zhao Y.; Qin R. X.; Mo S. G.; Chen G. X.; Gu L.; Chevrier D. M.; Zhang P.; Guo Q.; Zang D. D.; Wu B. H.; Fu G.; Zheng N. F. Science 2016, 352, 797. |
/
| 〈 |
|
〉 |