

Self-assembly of Supramolecular Planar Macrocycle Driven by Intermolecular Halogen Bonding
Received date: 2022-08-30
Online published: 2022-10-08
Supported by
Key Scientific Research Projects of Colleges and Universities of Henan Province(21A150045); Start-up Foundation for Scientific Research of Newly Recruited PhD of Shangqiu Normal University(700200); Start-up Foundation for Scientific Research of Newly Recruited PhD of Shangqiu Normal University(700205)
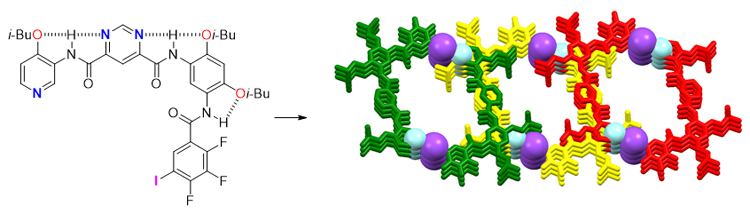
The design and synthesis of three kinds of arylamide molecules (compounds 1~3) containing halogen bonding donor and acceptor fragments, and the exploration and analyzation of different action modes of halogen bonding in solid phase were reported. Compounds 1 and 2 contain two tetrafluoroiodobenzene fragments, and compound 1 also contains a halogen receptor fragment—pyridine group. Isobutyl groups are introduced into the molecule to increase its solubility and crystallinity. And a pyrimidine fragment was introduced into compound 3, which has more aromatic rings. The two N atoms of the pyrimidine fragment can theoretically form intramolecular hydrogen bonds with the adjacent amide hydrogen atoms (—C(=O)NH), so that the whole molecule has the properties of hydrogen-bonded arylamide foldamer. Moreover, trifluorobenzene fragments were selected in compound 3 to eliminate the repulsion between excess fluorine atoms and carbonyls. The crystal structures reveal that the three aromatic rings in compound 1 are twisted with each other for there is no intramolecular hydrogen bond, and a supramolecular DNA-like double helix was assembled controlled by intermolecular N···I and O···I halogen bonds arranged alternately. Compound 2 failed to form an intramolecular three-center hydrogen bonding due to the repulsion between the amide carbonyl groups and the two fluorine atoms in close proximity. As expected, in the solid phase of compound 3, an effective three-center hydrogen bond is formed between the terminal trifluoroiodobenzene and the benzene ring attached to it. Moreover, the two N—H bonds connected to the pyrimidine ring also form two effective three-center hydrogen bonds. The difference is that the participants of these two groups of three-center hydrogen bonds include two N atoms in the pyrimidine ring. The four aromatic rings in compound 3 are nearly coplanar driven by these intramolecular three-center hydrogen bonds. Two sets of strong intermolecular (pyridine ring) N···I halogen bonds control the formation of [1+1] bimolecular supramolecular macrocycles with inner diameters of 1.36 nm and 1.07 nm in length and width. Moreover, the supramolecular macrocycle is near-planar due to the introduction of pyrimidine ring.
Chuanzhi Liu , Fen Li , Jingjing Wang , Xiaolu Zhao , Tingmei Zhang , Xin Huang , Mengli Wu , Zhiyuan Hu , Xinming Liu , Zhanting Li . Self-assembly of Supramolecular Planar Macrocycle Driven by Intermolecular Halogen Bonding[J]. Acta Chimica Sinica, 2022 , 80(10) : 1365 -1368 . DOI: 10.6023/A22080368
| [1] | Politzer, P.; Lane, P.; Concha, M. C.; Ma, Y.; Murray, J. S. J. Mol. Model. 2007, 13, 305. |
| [2] | Rissanen, K. CrystEngComm 2008, 10, 1107. |
| [3] | Priimagi, A.; Cavallo, G.; Metrangolo, P.; Resnati, G. Acc. Chem. Res. 2013, 46, 2686. |
| [4] | Mukherjee, A.; Tothadi, S.; Desiraju, G. R. Acc. Chem. Res. 2014, 47, 2514. |
| [5] | Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. Chem. Rev. 2016, 116, 2478. |
| [6] | Fu, Y.; Xiang, Z.; Zhou, J.; Wu, X.; Li, Y.; Jiao, Y. Acta Chim. Sinica 2012, 70, 1847 ( in chinese). |
| [6] | (付昱, 向子龙, 周军, 吴欣蔚, 李妍, 焦永华, 化学学报, 2012, 70, 1847.) |
| [7] | Liu, C.-Z.; Wang, H.; Zhang, D.-W.; Zhao, X.; Li, Z.-T. Chin. J. Org. Chem. 2019, 39, 28. (in Chinese) |
| [7] | (刘传志, 王辉, 张丹维, 赵新, 黎占亭, 有机化学, 2019, 39, 28.) |
| [8] | Ding, X.-H.; Chang, Y.-Z.; Ou, C.-Q.; Lin, J.-Y.; Xie, L.-H.; Huang, W. Natl. Sci. Rev. 2020, 7, 1906. |
| [9] | Gilday, L. C.; Robinson, S. W.; Barendt, T. A.; Langton, M. J.; Mullaney, B. R.; Beer, P. D. Chem. Rev. 2015, 115, 7118. |
| [10] | Kianimehra, A.; Akhbaria, K.; Whiteb, J.; Phuruangratc, A. Inorg. Chem. Commun. 2020, 115, 107864. |
| [11] | Bulfield, D.; Huber, S. M. Chem. Eur. J. 2016, 22, 14434. |
| [12] | Sutar, R. L.; Huber, S. M. ACS Catal. 2019, 9, 9622. |
| [13] | Zhang, H.-M.; Toya, P. H. Adv. Synth. Catal. 2021, 363, 215. |
| [14] | Heinen, F.; Reinhard, D. L.; Engelage, E.; Huber, S. M. Angew. Chem. Int. Ed. 2021, 60, 5069. |
| [15] | Ma, X.-K.; Zhang, W.; Liu, Z.; Zhang, H.; Zhang, B.; Liu, Y. Adv. Mater. 2021, 2007476. |
| [16] | Cao, J.; Yan, X.; He, W.; Li, X.; Li, Z.; Mo, Y.; Liu, M.; Jiang, Y.-B. J. Am. Chem. Soc. 2017, 139, 6605. |
| [17] | Zheng, J.; Suwardi, A.; Wong, C. J. E.; Loh, X. J.; Li, Z. B. Nanoscale Adv. 2021, 3, 6342. |
| [18] | Gong, G.-F.; Lv, S.-H.; Han, J.-X.; Xie, F.; Li, Q.; Xia, N.; Zeng, W.; Chen, Y.; Wang, L.; Wang, J.-K.; Chen, S.-G. Angew. Chem. Int. Ed. 2021, 60, 14831. |
| [19] | Borchers, T.-H.; Topić, F.; Christopherson, J. C.; Bushuyev, O.-S.; Vainauskas, J.; Titi, H. M.; Friščić, T.; Barrett, C. J. Nature Chem. 2022, 14, 574. |
| [20] | Montaña, Á. M. ChemistrySelect 2017, 2, 9094. |
| [21] | Sumii, Y.; Sasaki, K.; Tsuzuki, S.; Shibata, N. Molecules 2019, 24, 3610. |
| [22] | Zhu, Z.; Wang, G.; Xu, Z.; Chen, Z.; Wang, J.; Shi, J.; Zhu, W. Phys. Chem. Chem. Phys. 2019, 21, 15106. |
| [23] | Zhao, H.; Sheng, S.; Hong, Y.; Zeng, H. J. Am. Chem. Soc. 2014, 136, 14270. |
| [24] | Jentzsch, A. V.; Matile, S. Top. Curr. Chem. 2014, 358, 205. |
| [25] | Huo, Y. P.; Zeng, H. Q. Acc. Chem. Res. 2016, 49, 922. |
| [26] | Shen, J.; Fan, J. R.; Ye, R. J.; Li, N.; Mu, Y. G.; Zeng, H. Q. Angew. Chem. Int. Ed. 2020, 59, 13328. |
| [27] | Zheng, S.-P.; Huang, L.-B.; Sun, Z.-H.; Barboiu, M. Angew. Chem. Int. Ed. 2021, 60, 566. |
| [28] | Wang, C.-X.; Wang, S.-K.; Yang, H.-T.; Xiang, Y.-X.; Wang, X.-B.; Bao, C.-Y.; Zhu, L.-Y.; Tian, H.; Qu, D.-H. Angew. Chem. Int. Ed. 2021, 60, 14836. |
| [29] | Chen, S.-J.; Wang, Y.-C.; Nie, T.; Bao, C.-Y.; Wang, C.-X.; Xu, T.-Y.; Lin, Q.-N.; Qu, D.-H.; Gong, X.-Q.; Yang, Y.; Zhu, L.-Y.; Tian, H. J. Am. Chem. Soc. 2018, 140, 17992. |
| [30] | Pancholi, J.; Beer, P. D. Coord. Chem. Rev. 2020, 416, 213281. |
| [31] | Dong, S. Y.; Zheng, B.; Wang, F.; Huang, F. H. Acc. Chem. Res. 2014, 47, 1982. |
| [32] | Jungbauer, S. H.; Bulfield, D.; Kniep, F.; Lehmann, C. W.; Herdtweck, E.; Huber, S. M. J. Am. Chem. Soc. 2014, 136, 16740. |
| [33] | Gong, B. Chem.-Eur. J. 2001, 7, 4336. |
| [34] | Huc, I. Eur. J. Org. Chem. 2004, 17. |
| [35] | Li, Z.-T.; Hou, J.-L.; Li, C. Acc. Chem. Res. 2008, 41, 1343. |
| [36] | Roy, A.; Prabhakaran, P.; Baruah, P. K.; Sanjayan, G. J. Chem. Commun. 2011, 47, 11593. |
| [37] | Zhang, D.-W.; Zhao, X.; Hou, J.-L.; Li, Z.-T. Chem. Rev. 2012, 112, 5271. |
| [38] | Liu, C.-Z.; Yan, M.; Wang, H.; Zhang, D.-W.; Li, Z.-T. ACS Omega 2018, 3, 5165. |
| [39] | Sun, G.-J.; Nie, C.-B.; Zhao, X.; Li, Z.-T. Chin. J. Org. Chem. 2017, 37, 1757. (in Chinese) |
| [39] | (孙广军, 聂承斌, 赵新, 黎占亭, 有机化学, 2017, 37, 1757.) |
| [40] | Yang, L.; Zhao, W.; Che, Y.-K.; Wang, Y.; Jiang, H. Chin. Chem. Lett. 2017, 28, 1659. |
| [41] | Zhang, D.-W.; Wang, H.; Li, Z.-T. Macromol. Rapid Commun. 2017, 38, 1700179. |
| [42] | Liu, C.-Z.; Koppireddi, S.; Wang, H.; Zhang, D.-W.; Li, Z.-T. Angew. Chem. Int. Ed. 2019, 58, 226. |
| [43] | Liu, C.-Z.; Koppireddi, S.; Wang, H.; Zhang, D.-W.; Li, Z.-T. Chin. Chem. Lett. 2019, 30, 953. |
| [44] | Koppireddi, S.; Liu, C.-Z.; Wang, H.; Zhang, D.-W.; Li, Z.-T. CrystEngComm 2019, 21, 2626. |
| [45] | Xu, Y.-Y.; Liu, C.-Z.; Wang, H.; Zhang, D.-W.; Li, Z.-T. Chin. J. Org. Chem. 2021, 41, 2848. (in Chinese) |
| [45] | (许艳艳, 刘传志, 王辉, 张丹维, 黎占亭, 有机化学, 2021, 41, 2848.) |
| [46] | Yu, S.; Kalenius, E.; Frontera, A.; Rissanena, K. Chem. Commun. 2021, 57, 12464. |
| [47] | Metrangolo, P.; Meyer, F.; Pilati, T.; Resnati, G.; Terraneo, G. Angew. Chem. Int. Ed. 2008, 47, 6114. |
| [48] | Gilday, L. C.; Robinson, S. W.; Barendt, T. A.; Langton, M. J.; Mullaney, B. R.; Beer, P. D. Chem. Rev. 2015, 115, 7118. |
| [49] | Wang, H.; Wang, W.; Jin, W.-J. Chem. Rev. 2016, 116, 5072. |
| [50] | Tepper, R.; Schubert, U. S. Angew. Chem. Int. Ed. 2018, 57, 6004. |
| [51] | Jiang, H.; Léger, J. M.; Huc, I. J. Am. Chem. Soc. 2003, 125, 3448. |
/
| 〈 |
|
〉 |