

One-Pot Synthesis of 1,4-Bridged Dihydroisoquinoline-3-ones from Isoquinolinium Salts and Cyclic 1,3-Diketones
Received date: 2022-09-30
Online published: 2022-11-11
Supported by
Natural Science Foundation of Chongqing(cstc2017jcyjAX0423)
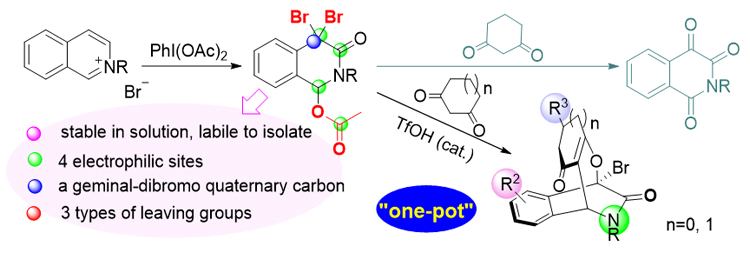
Bridged isoquinoline derivatives play an important role in various bioactive molecules. The cascade dearomatizative annulation of isoquinolinium salts with bis-nucleophiles is a straightforward strategy to construct bridged isoquinoline skeletons because isoquinolinium ions have two electrophilic sites. However, the reported examples only focused on the synthesis of 1,3-bridged cyclic skeletons. In the previous work, it was reported the first synthesis of 1,4-bridged dihydroisoquinolin-3-ones from isoquinolinium salts and 4-hydroxycoumarins. When cyclic 1,3-diketones were used instead of 4-hydroxycoumarins, isoquinoline-1,3,4(2H)-triones, instead of the expected 1,4-bridged dihydroisoquinolin-3-ones, were unexpectedly yielded. Experimental evidence by high resolution mass spectroscopy supports that the generation of isoquinoline-1,3,4(2H)-triones was initiated via an O-nucleophilic substitution of the cyclic 1,3-diketone, followed by an elimination of the 2-bromo-cyclic 1,3-diketone to give intermediate 4-bromoisoquinolin-3(2H)-one, which subsequently underwent dual hydrolyses and aerobic oxidations. Based on this mechanism, the O-nucleophilic substitution of cyclic 1,3-diketones was successfully inhibited by the addition of a catalytic amount of trifluoromethanesulfonic acid (TfOH). The desired 1,4-bridged dihydroisoquinolin-3-ones were then obtained. This method provides a facile access to 1,4-bridged isoquinoline skeletons under mild reaction conditions (33 examples). The general procedure is as following: under an argon atmosphere, a 5 mL Schlenk flask was charged with isoquinolinium salt 6 (0.2 mmol), phenyliodine(III) diacetate (0.6 mmol), KBr (0.2 mmol), H2O (3.6 μL), and dry dichloroethane (2 mL). The mixture was continually stirred at room temperature until 6 was consumed as indicated by thin-layer chromatography (TLC). TfOH (5 μL) and cyclic 1,3-diketone 7 (0.4 mmol) were sequentially added. The reaction mixture was heated at 50 ℃ in the oil bath until the intermediate was consumed as indicated by TLC, then cooled to room temperature, diluted with water (5 mL), and extracted with ethyl acetate (5 mL×3). The combined organic layer was washed with brine, dried over Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel (petroleum ether/EtOAc as the eluent) to give the 1,4-bridged product 8.
Yucheng Yin , Lijing Leng , Xiaolong Lin , Yan Yu , Tian Cai , Qunli Luo . One-Pot Synthesis of 1,4-Bridged Dihydroisoquinoline-3-ones from Isoquinolinium Salts and Cyclic 1,3-Diketones[J]. Acta Chimica Sinica, 2022 , 80(12) : 1569 -1575 . DOI: 10.6023/A22090408
| [1] | (a) Li, K.; Ou, J.-J.; Gao, S. Angew. Chem., Int. Ed. 2016, 55, 14778. |
| [1] | (b) Wang, B.-X.; Xu, Z.-X.; Wu, J. Chin. J. Org. Chem. 2006, 26, 1587. (in Chinese) |
| [1] | 王炳祥, 徐助雄, 吴婧, 有机化学 2006, 26, 1587.) |
| [1] | (c) Shi, L.; Ji, Y.; Huang, W.-X.; Zhou, Y.-G. Acta Chim. Sinica 2014, 72, 820. (in Chinese) |
| [1] | ( 时磊, 姬悦, 黄文学, 周永贵, 化学学报 2014, 72, 820.) |
| [2] | Capacci, A. G.; Dechantsreiter, M.; Enyedy, I.; Jones, J. H.; Lin, E. Y.-S.; Lucas, B. S.; Ma, B. WO 2018140876, 2018 |
| [2] | [Chem. Abstr. 2018, 169, 242866 ] |
| [3] | Tanifuji, R.; Minami, A.; Oguri, H.; Oikawa, H. Nat. Prod. Rep. 2020, 37, 1098. |
| [4] | Krishnan, S.; Bagdanoff, J. T.; Ebner, D. C.; Ramtohul, Y. K.; Tambar, U. K.; Stoltz, B. M. J. Am. Chem. Soc. 2008, 130, 13745. |
| [5] | Le, V. H.; Inai, M.; Williams, R. M.; Kan, T. Nat. Prod. Rep. 2015, 32, 328. |
| [6] | (a) Ding, B.-D.; Jiang, Y.-C.; Zhang, Y.; Ye, R.; Sun, J.; Yan, C.-G. Chin. J. Org. Chem. 2020, 40, 1003. (in Chinese) |
| [6] | ( 丁邦东, 姜业朝, 张瑜, 叶蓉, 孙晶, 颜朝国, 有机化学 2020, 40, 1003.) |
| [6] | (b) Shen, G.-L.; Sun, J.; Yan, C.-G. Chin. J. Chem. 2016, 34, 412. |
| [6] | (c) Niu, F.; Cui, Z.; Chang, H.-T.; Jiang, Y.; Chen, F.-K.; Tu, P.-F. Chin. J. Chem. 2006, 24, 1788. |
| [6] | (d) Wang, L.-L.; Zhang, Z.-Y.; Han, H.-B.; Liu, X.-L.; Bu, Z.-W.; Wang, Q.-L. Chin. J. Org. Chem. 2021, 41, 12. (in Chinese) |
| [6] | ( 王乐乐, 张子莹, 韩华彬, 刘雄利, 卜站伟, 王琪琳, 有机化学 2021, 41, 12.) |
| [6] | (e) Das, S. Org. Biomol. Chem. 2022, 20, 1838. |
| [6] | (f) Zhang, Z.-Y.; Han, H.-B.; Wang, L.-L.; Bu, Z.-W.; Xie, Y.; Wang, Q.-L. Org. Biomol. Chem. 2021, 19, 3960. |
| [6] | (g) Sharma, U. K.; Ranjan, P.; Van der Eycken, E. V.; You, S.-L. Chem. Soc. Rev. 2020, 49, 8721. |
| [6] | (h) Xu, J.-H.; Zheng, S.-C.; Zhang, J.-W.; Liu, X.-Y.; Tan, B. Angew. Chem., Int. Ed. 2016, 55, 11834. |
| [6] | (i) Bai, X.-G.; Miao, H.-J.; Zhao, Y.; Wang, Q.-L.; Bu, Z.-W. Org. Lett. 2020, 22, 5068. |
| [6] | (j) Miao, H.-J.; Wang, L.-L.; Han, H.-B.; Zhao, Y.-D.; Wang, Q.-L.; Bu, Z.-W. Chem. Sci. 2020, 11, 1418. |
| [6] | (k) Miao, H.-J.; Bai, X.-G.; Wang, L.-L.; Yu, J.-H.; Wang, Q.-L.; Bu, Z.-W. Org. Chem. Front. 2021, 8, 204. |
| [7] | Lin, X.-L.; Yu, Y.; Zhang, L.; Leng, L.-J.; Xiao, D.-R.; Cai, T.; Luo, Q.-L. Org. Chem. Front. 2022, 9, 4676. |
| [8] | For details, see the Supporting Information. |
| [9] | Increasing the reaction temperature did not improve the yield of isoquinoline-1,3,4-triones (also see the section of 2.2.1). Therefore, O-SN1 could be ruled out. |
| [10] | Bai, L.-G.; Zhou, Y.; Zhuang, X.; Zhang, L.; Xue, J.; Lin, X.-L.; Cai, T.; Luo, Q.-L. Green Chem. 2020, 22, 197. |
| [11] | Wang, S.-K.; Chen, M.-T.; Zhao, D.-Y.; You, X.; Luo, Q.-L. Adv. Synth. Catal. 2016, 358, 4093. |
/
| 〈 |
|
〉 |