

Enantiomers Identification of Penicillamine by Chiral Mono-Schiff Base Macrocycles
Received date: 2022-09-25
Online published: 2022-12-12
Supported by
National Natural Science Foundation of China(21961007)
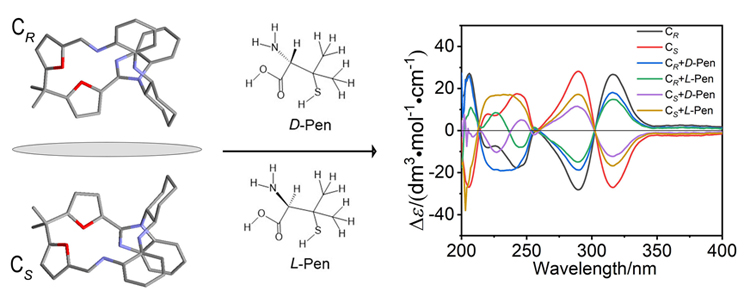
A large number of chiral drug molecules have a chiral structure containing both D- and L-enantiomers, leading to the mirror image arrangements of the two forms eliciting quite different physiological responses. Penicillamine (Pen) is a common chiral drug that is obtained from penicillin, and researchers have been making continuous efforts to achieve rigorous analysis and discrimination of the two enantiomers. Based on chiral Schiff base macrocycles containing NH functionalities have the advantages of mild synthesis conditions and convergence of structural arrangement. Here we report two enantiomers of the mono-Schiff base macrocycle containing chiral NH moiety in the cyclic structure (CR and CS), and the binding affinity and enantioselectivity of the cyclic enantiomers toward small molecules penicillamine (D-Pen and L-Pen). The crystal structures of the mono-Schiff base cyclic enantiomers were determined by X-ray diffraction analysis, and the results show that the two cyclic enantiomers exhibit twisted non-planar conformation, in which the chiral NH proton points to the inside of the ring cavity. The interactions between different enantiomers of the chiral macrocycle and penicillamine were investigated by ultraviolet visible (UV-Vis) and hydrogen nuclear magnetic resonance (1H NMR) titration, and the results show that the chiral macrocycle binds with the enantiomers of penicillamine with a bonding ratio of 1∶1, binding constants around 107 L•mol-1, and the complexes of [C-Pen+H]+ can be easily formed and detected by electrospray ionization-mass spectrometry (ESI-MS). The interactions between different enantiomers of the chiral macrocycle and penicillamine are attributed to the intermolecular hydrogen bonding of enantiomers by the asymmetrical chiral NH moiety in the mono-Schiff base macrocycle. Comparison of bonding constants based on the chiral macrocycle binds with the enantiomers of penicillamine, the results show that the chiral CR exhibits higher enantioselectivity for L-Pen, while CS exhibits the higher enantioselectivity for D-Pen, with a binding constant ratio around 2, respectively. Further, investigation of circular dichroism (CD) spectroscopic titration indicate that the penicillamine with the same chirality as the host macrocycle binds stronger with the host than its enantiomer with the host.
Xiaomao Tian , Yuequn Lin , Han Zhu , Chao Huang , Bixue Zhu . Enantiomers Identification of Penicillamine by Chiral Mono-Schiff Base Macrocycles[J]. Acta Chimica Sinica, 2023 , 81(1) : 20 -28 . DOI: 10.6023/A22090400
| [1] |
Xiao, T.; Li, S.; Zhang, X.; Lin, C.; Wang, L. Y. Chin. J. Chem. 2013, 31, 627.
|
| [2] |
Geer, M. F.; Walla, M.; Solntsev, K.; Strassert, C.; Shimizu, L. S. J. Org. Chem. 2013, 78, 5568.
|
| [3] |
Radujevi?, A.; Penavic, A.; Pavlovi?, R. Z.; Badji?, J. D.; Anzenbacher, P. Chem 2022, 8, 2228.
|
| [4] |
Wang, D.; You, L.; Wang, J.; Wang, H.; Zhang, D.; Li, Z. T. Tetrahedron Lett. 2013, 54, 6967.
|
| [5] |
María, D.; Claramunt, R.; Torralba, M.; Torres, M.; Elguero, J. Tetrahedron Lett. 2019, 60, 1206.
|
| [6] |
Wei, X. K.; Gu, J. C.; Liu, X. L.; Huang, C.; Zhu, B. X. Chin. J. Org. Chem. 2018, 38, 3386. (in Chinese)
|
| [6] |
( 魏小康, 谷静池, 刘兴丽, 黄超, 朱必学, 有机化学, 2018, 38, 3386.)
|
| [7] |
Xiong, Y.; Huang, C.; Liu, H. J.; Yi, R.; Zhu, B. X.; Ni, X. L. Chin. Chem. Lett. 2021, 32, 3522.
|
| [8] |
Li, D. H.; Smith, B. D. Beilstein J. Org. Chem. 2019, 15, 1086.
|
| [9] |
Barisic, D.; Lesic, F.; Vlasic, M. T.; Uzarevic, K.; Bregovic, N.; Tomisi, V. Tetrahedron 2022, 120, 132875.
|
| [10] |
Tromans, R. A.; Carter, T. S.; Chabanne, L.; Crump, M. P.; Li, H. Y.; Matlock, J. V.; Orchard, M. G.; Davis, A. P. Nat. Chem. 2019, 11, 52.
|
| [11] |
Roth, J. Chem. Rev. 2002, 102, 285.
|
| [12] |
Glavin, D. P.; Burton, A. S.; Elsila, J. E.; Aponte, J. C.; Dworkin, J. P. Chem. Rev. 2019, 120, 4660.
|
| [13] |
Lu, H. J.; Yu, C. T.; Guo, Y. L. Acta Chim. Sinica 2002, 60, 882. (in Chinese)
|
| [13] |
( 陆豪杰, 余翀天, 郭寅龙, 化学学报, 2002, 60, 882.)
|
| [14] |
Li, D.; Gao, B. J.; Xu, W. M. Acta Chim. Sinica 2011, 69, 3019 (in Chinese)
|
| [14] |
( 李丁, 高保娇, 许文梅, 化学学报, 2011, 69, 3019.)
|
| [15] |
Liu, Z.; Zhang, S.; Cheng, M.; Yang, L.; Li, G.; Xu, W. W.; Qu, H. N.; Liang, F.; Cheng, J.; Li, H. B. Analyst 2022, 147, 1803.
|
| [16] |
Zhu, B. X.; Ruan, W. J.; Gao, F.; Hu, G. H.; Zhu, Z. A. Acta Chim. Sinica 2004, 62, 58. (in Chinese)
|
| [16] |
( 朱必学, 阮文娟, 高峰, 胡国航, 朱志昂, 化学学报, 2004, 62, 58.)
|
| [17] |
Liu, T.; Ruan, W. J.; Nan, J.; Zhu, Z. A. Chin. J. Chem. 2003, 21, 751.
|
| [18] |
Forte, G.; Sfrazzetto, G. T.; Pappalardo, A. Comput. Theor. Chem. 2015, 1068, 8.
|
| [19] |
Ikbal, S. A.; Sakata, Y.; Akine, S. Dalton Trans. 2021, 50, 4119.
|
| [20] |
Peluso, P.; Chankvetadze, B. Chem. Rev. 2022, 122, 13235.
|
| [21] |
Rajasekar, P.; Jose, C.; Sarkar, M.; Boomishankar, R. Angew. Chem. Int. Ed. 2021, 60, 4023.
|
| [22] |
Wang, S. Y.; Li, L.; Xiao, Y.; Wang, Y. Trends Anal. Chem. 2019, 121, 115691.
|
| [23] |
Padovani, D.; Galardon, E. Chem. Res. Toxicol. 2022, 35, 412.
|
| [24] |
Durán, G. M.; Abellán, C.; Contento, A. M.; Ríos, á. Microchim. Acta 2017, 184, 815.
|
| [25] |
Mendes, J.; Almeida, K. J.; Neto, J. L.; Ramalho, T. C.; Duarte, H. A. J. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2017, 184, 308.
|
| [26] |
Yang, N.; Zhang, K.; Guan, Q. W.; Wang, Z. J.; Chen, K. N.; Mao, X. Y. Antioxidants 2022, 11, 1602.
|
| [27] |
Zhang, Y.; Wang, H. Y.; He, X. W.; Li, W. Y.; Zhang, Y. K. J. Hazard. Mater. 2021, 412, 125249.
|
| [28] |
Sun, L.; Huang, F.; Liu, W. W.; Lin, L.; Hong, Y.; Kong, X. L. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2020, 241, 118653.
|
| [29] |
Li, Z. B.; Lin, J.; Pu, L. Angew. Chem. Int. Ed. 2005, 44, 1690.
|
| [30] |
Zhu, Y. Y.; Wu, X. D.; Gu, S. H.; Pu, L. J. Am. Chem. Soc. 2019, 141, 175.
|
| [31] |
Pu, L. Chem. Commun. 2022, 58, 8038.
|
| [32] |
Dong, J. Q.; Tan, C. X.; Zhang, K.; Liu, Y.; Low, P. J.; Jiang, J. W.; Cui, Y. J. Am. Chem. Soc. 2017, 139, 1554.
|
| [33] |
Gangemi, C. M. A.; Rimkaite, U.; Cipria, F.; Sfrazzetto, G. T.; Pappalardo, A. Front. Chem. 2019, 7, 836.
|
| [34] |
Yang, S. T.; Wu, F. L.; Yu, F. Z.; Gu, L. C.; Wang, H. H.; Liu, Y. Y.; Chu, Y. Q.; Wang, F. Y.; Fang, X.; Ding, C. F. Anal. Chim. Acta 2021, 1184, 339017.
|
| [35] |
Ema, T.; Yamasaki, T.; Watanabe, S.; Hiyoshi, M.; Takaishi, K. J. Org. Chem. 2018, 83, 10762.
|
| [36] |
Wen, J.; Feng, L.; Zhao, H. M.; Zheng, L.; Stavropoulos, P.; Ai, L.; Zhang, J. X. J. Org. Chem. 2022, 87, 7934.
|
| [37] |
Ohishi, Y.; Chiba, J.; Inouye, M. J. Org. Chem. 2022, 87, 10825.
|
| [38] |
Thordarson, P. Chem. Soc. Rev. 2011, 40, 1305.
|
| [39] |
Shi, M.; Qian, H. X. Tetrahedron 2005, 61, 4949.
|
| [40] |
Chen, Z. H.; Wu, C. T. Chin. J. Org. Chem. 2002, 22, 582. (in Chinese)
|
| [40] |
( 陈展虹, 吴成泰, 有机化学, 2002, 22, 582.)
|
/
| 〈 |
|
〉 |