

Difluoroallylation of Silanes under Photoirradiation
Received date: 2022-11-09
Online published: 2023-01-04
Supported by
National Natural Science Foundation of China(21961045); National Natural Science Foundation of China(22061048); Yunnan Provincial Key Laboratory Construction Plan Funding of Universities and Yunnan Provincial Engineering Research Center Construction Plan Funding of Universities
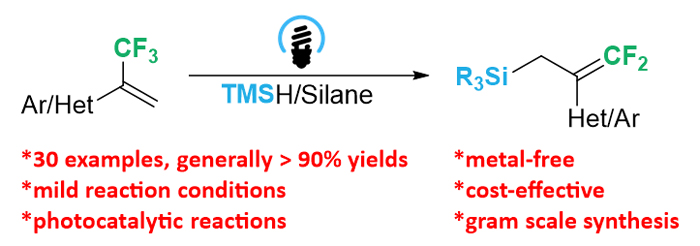
The synthesis of organosilicon compounds has attracted considerable interest, especially the allyl silanes, which are regarded as ideal building blocks in the synthesis of small molecules and polymers. The traditional synthetic method for allyl silanes relies on the cross-coupling of Grignard reagent and chlorosilane (Silyl-Kumada reaction), transition metal catalyzed silylation of allylic alcohols with disilanes or silylboranes, and regioselective silylation of conjugated alkenes or allenes. Although some other methods were also developed, the using of transition metal catalysts has resulted in disadvantages such as contamination of desired allyl silanes and high production costs. Therefore, a mild and metal-free practical method is highly desired. We herein describe a metal-free difluoroallylation of silanes with α-trifluoromethyl alkenes in the presence of quinuclidine as hydrogen atom transfer (HAT) reagent under the irradiation of 30 W blue light-emitting diode (LED) (460~470 nm) at room temperature. To an oven dried Schlenk-tube, trifluoromethylpropene (0.1 mmol), silane (0.3 mmol), KHCO3 (0.1 mmol), 4-CzIPN (1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene) (0.002 mmol) and MeCN (1 mL) were added under argon atmosphere. The reaction mixture was stirred under the irradiation of 30 W blue LED (460~470 nm) at room temperature. After completion of the reaction, the reaction solution was removed by reduced pressure and the residue was purified by silica gel chromatographic column to obtain gem-difluoroallylation products. A wide range of aromatic and heterocyclic α-trifluoromethyl alkenes were successfully applied in the difluoroallylation of silanes to afford difluoroallylsilanes. And all of the tested silanes, including trimethylsilane, sterically more demanding silanes as well as dimethylphenylsilane all participated in the present transformation readily to afford the corresponding difluoroallylsilanes in excellent yields. The present methodology has provided an efficient and cost-effective gram scale synthetic method for the preparation of difluoroallylsilanes under blue light irradiation in the presence of 4-CzIPN as organic photosensitizer. The scalability in large scale and excellent functional group compatibility of this transformation ensures broad applicability to a variety of difluoroallylsilanes. The proposed reaction mechanism showed that the reaction proceeded through the radical addition of α-trifluoromethyl alkene with silane free radical and subsequent β-fluoride elimination.
Chunhui Yang , Jingchao Chen , Xinhan Li , Li Meng , Kaimin Wang , Weiqing Sun , Baomin Fan . Difluoroallylation of Silanes under Photoirradiation[J]. Acta Chimica Sinica, 2023 , 81(1) : 1 -5 . DOI: 10.6023/A22110454
| [1] | Chen, C.-A.; Sieburth, S. M.; Glekas, A.; Hewitt, G. W.; Trainor, G. L.; Erickson-Viitanen, S.; Garber, S. S.; Cordova, B.; Jeffry, S.; Klabe, R. M. Chem. Biol. 2001, 8, 1161. |
| [2] | Mutahi, M. W.; Nittoli, T.; Guo, L.-X.; Sieburth, S. M. J. Am. Chem. Soc. 2002, 124, 7363. |
| [3] | Gately, S.; West, R. Drug Dev. Res. 2007, 68, 156. |
| [4] | Franz, A. K.; Wilson, S. O. J. Med. Chem. 2013, 56, 388. |
| [5] | Tacke, R.; Popp, F.; Müller, B.; Theis, B.; Burschka, C.; Hamacher, A.; Kassack, M. U.; Schepmann, D.; Wünsch, B.; Jurva, U.; Wellner, E. ChemMedChem 2008, 3, 152. |
| [6] | For reviews and recent examples, see: (a) Wang, D.; Chen, D.-H. Acta Chim. Sinica 1990, 48, 516. (in Chinese) |
| [6] | ( 王东, 陈德恒, 化学学报, 1990, 48, 516). |
| [6] | (b) Fleming, I.; Barbero, A.; Walter, D. Chem. Rev. 1997, 97, 2063. |
| [6] | (c) Hiyama, T. J. Organomet. Chem. 2002, 653, 58. |
| [6] | (d) Chabaud, L.; James, P.; Landais, Y. Eur. J. Org. Chem. 2004, 15, 3173. |
| [6] | (e) Komiyama, T.; Minami, Y.; Hiyama, T. ACS Catal. 2017, 7, 631. |
| [7] | For reviews and recent examples, see: (a) Tseng, C.-L.; Zhuo, R.-X.; Liu, J.-W. Acta Chim. Sinica 1964, 30, 360. (in Chinese) |
| [7] | ( 曾昭抡, 卓仁禧, 刘基万, 化学学报, 1964, 30, 360). |
| [7] | (b) Chan, T. H.; Wang, D. Chem. Rev. 1995, 95, 1279. |
| [7] | (c) Langkopf, E.; Schinzer, D. Chem. Rev. 1995, 95, 1375. |
| [7] | (d) Barbero, A.; Pulido, F. J. Acc. Chem. Res. 2004, 37, 817. |
| [7] | (e) Nielsen, L.; Skrydstrup, T. J. Am. Chem. Soc. 2008, 130, 13145. |
| [7] | (f) Hu, Y.-Q.; Huang, D.-F.; Wang, K.-H.; Zhao, Z.-X.; Zhao, F.-X.; Xu, W.-G.; Hu, Y.-L. Chin. J. Org. Chem. 2020, 40, 1689. (in Chinese) |
| [7] | 虎永琴, 黄丹凤, 王克虎, 赵转霞, 赵芳霞, 徐炜刚, 胡雨来, 有机化学, 2020, 40, 1689). |
| [8] | For recent examples, see: (a) Murakami, K.; Yorimitsu, H.; Oshima, K. J. Org. Chem. 2009, 74, 1415. |
| [8] | (b) Selander, N.; Paasch, J. R.; Szabó, K. J. J. Am. Chem. Soc. 2011, 133, 409. |
| [8] | (c) Vulovic, B.; Cinderella, A. P.; Watson, D. A. ACS Catal. 2017, 7, 8113. |
| [8] | (d) Larsson, J. M.; Szabó, K. J. J. Am. Chem. Soc. 2013, 135, 443. |
| [8] | (e) Gan, Y.; Xu, W.; Liu, Y.-H. Org. Lett. 2019, 21, 9652. |
| [8] | (f) Yang, B.; Wang, Z.-X. Org. Lett. 2019, 21, 7965. |
| [9] | For recent examples, see: (a) Selander, N.; Paasch, J. R.; Szabó, K. J. J. Am. Chem. Soc. 2011, 133, 409. |
| [9] | (b) Larsson, J. M.; Szabó, K. J. J. Am. Chem. Soc. 2013, 135, 443. |
| [9] | (c) Yang, B.; Wang, Z.-X. Org. Lett. 2019, 21, 7965. |
| [9] | (d) Gan, Y.; Xu, W.; Liu, Y.-H. Org. Lett. 2019, 21, 9652. |
| [10] | For recent examples, see: (a) Ohmura, T.; Taniguchi, H.; Suginome, M. J. Am. Chem. Soc. 2006, 128, 13682. |
| [10] | (b) Miller, Z. D.; Li, W.; Belderrain, T. R.; Montgomery, J. J. Am. Chem. Soc. 2013, 135, 15282. |
| [10] | (c) Miller, Z. D.; Dorel, R.; Montgomery, J. Angew. Chem. Int. Ed. 2015, 54, 9088. |
| [10] | (d) Yeung, K.; Ruscoe, R. E.; Rae, J.; Pulis, A. P.; Procter, D. J. Angew. Chem. Int. Ed. 2016, 55, 11912. |
| [10] | (e) Da, B.-C.; Liang, Q.-J.; Luo, Y.-C.; Ahmad, T.; Xu, Y.-H.; Loh, T.-P. ACS Catal. 2018, 8, 6239. |
| [10] | (f) Da, B.-C.; Liang, Q.-J.; Luo, Y.-C.; Ahmad, T.; Xu, Y.-H.; Loh, T.-P. ACS Catal. 2018, 8, 6239. |
| [10] | (g) Sang, H.-L.; Yu, S.-J.; Ge, S.-Z. Chem. Sci. 2018, 9, 973. |
| [10] | (h) Zeng, J.-H.; Chen, J.-J.; Chen, L.; Zhan, Z.-P. Org. Chem. Front. 2020, 7, 1132. |
| [10] | (i) Xu, J.-L.; Xu, Z.-Y.; Wang, Z.-L.; Ma, W.-W.; Sun, X.-Y.; Fu, Y.; Xu, Y.-H. J. Am. Chem. Soc. 2022, 144, 5535. |
| [11] | (a) Takeda, M.; Shintani, R.; Hayashi, T. J. Org. Chem. 2013, 78, 5007. |
| [11] | (b) Xiao, Y.-L.; Pan, Q.; Zhang, X.-G. Acta Chim. Sinica 2015, 73, 383. (in Chinese) |
| [11] | ( 肖玉兰, 潘强, 张新刚, 化学学报, 2015, 73, 383). |
| [11] | (c) Mata, S.; López, L. A.; Vicente, R. Angew. Chem. Int. Ed. 2017, 56, 7930. |
| [11] | (d) Hofstra, J. L.; Cherney, A. H.; Ordner, C. M.; Reisman, S. E. J. Am. Chem. Soc. 2018, 140, 139. |
| [11] | (e) Zhao, S.; Li, C.-P.; Xu, B.; Liu, H. Chin. J. Org. Chem. 2020, 40, 1549. (in Chinese) |
| [11] | ( 赵森, 李淳朴, 许斌, 柳红, 有机化学, 2020, 40, 1549). |
| [12] | (a) Yue, F.-Y.; Liu, J.-H.; Ma, H.-N.; Liu, Y.-X.; Dong, J.-Y.; Wang, Q.-M. Org. Lett. 2022, 24, 4019. |
| [12] | (b) Luo, C.; Zhou, Y.; Chen, H.; Wang, T.; Zhang, Z.-B.; Han, P.; Jing, L.-H. Org. Lett. 2022, 24, 4286. |
| [12] | (c) Xu, W.-G.; Xia, C.-J.; Shao, Q.; Zhang, Q.; Liu, M.-R.; Zhang, H.-W.; Wu, M.-B. Org. Chem. Front. 2022, 9, 4949. |
| [13] | (a) Zhou, Q.-Q.; Düsel, S. J. S.; Lu, L.-Q.; K?nig, B.; Xiao, W.-J. Chem. Commun. 2019, 55, 107. |
| [13] | (b) Yu, X.-Y.; Chen, J.-R.; Xiao, W.-J. Chem. Rev. 2021, 121, 506. |
| [14] | (a) Li, K.-K.; Zhang, X.-X.; Chen, J.-C.; Gao, Y.; Yang, C.-H.; Zhang, K.-Y.; Zhou, Y.-Y.; Fan, B.-M. Org. Lett. 2019, 21, 9914. |
| [14] | (b) Zhang, X.-X.; Chen, J.-C.; Gao, Y.; Li, K.-K.; Zhou, Y.-Y.; Sun, W.-Q.; Fan, B.-M. Org. Chem. Front. 2019, 6, 2410. |
| [14] | (c) Li, K.-K.; Chen, J.-C.; Yang, C.-H.; Zhang, K.-Y.; Pan, C.-X.; Fan, B.-M. Org. Lett. 2020, 22, 4261. |
| [14] | (d) Lv, H.-P.; Laishram, R. D.; Chen, J.-C.; Khan, R.; Zhu, Y.-B.; Wu, S.-Y.; Zhang, J.-Q.; Liu, X.-Y.; Fan, B.-M. Chem. Commun. 2021, 57, 3660. |
| [15] | (a) Zhang, X.-H.; MacMillan, D. W. C. J. Am. Chem. Soc. 2017, 139, 11353. |
| [15] | (b) Le, C.; Liang, Y.-F.; Evans, R. W.; Li, X.-M.; MacMillan, D. W. C. Nature 2017, 547, 79. |
| [15] | (c) Hou, J.; Ee, A.; Cao, H.; Ong, H.-W.; Xu, J.-H.; Wu, J. Angew. Chem. Int. Ed. 2018, 57, 17220. |
| [15] | (d) Lei, G.-Y.; Xu, M.-C.; Chang, R.; Funes-Ardoiz, I.; Ye, J.-T. J. Am. Chem. Soc. 2021, 143, 11251. |
/
| 〈 |
|
〉 |