

A Gourd-shaped Organometallic Coordination Cage: Synthesis and Selective Binding of Two Drug Molecules
Received date: 2022-12-27
Online published: 2023-03-01
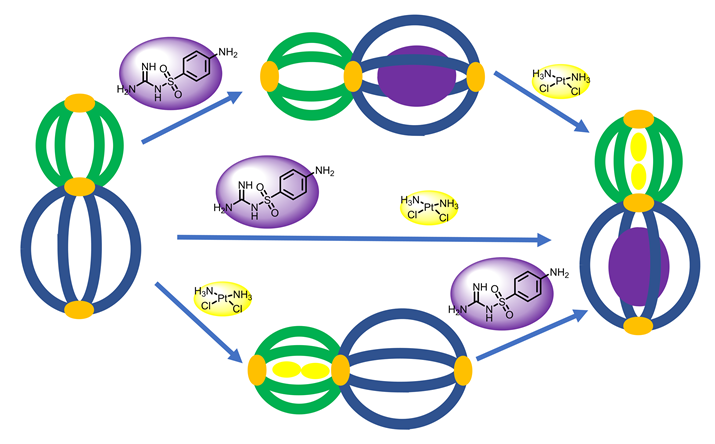
The Pd2L4 type coordination cage has been extensively studied and widely applied in catalysis, drug delivery, molecular recognition and other research fields since the seminal report in 1998. In this work, a gourd-shaped Pd2L4 type coordination cage (C1) was synthesized and characterized by proton nuclear magnetic resonance spectroscopy (1H NMR) and high resolution mass spectrum (HR-MS). The host-guest chemistry of C1 was studied by 1H NMR with two drug molecules, sulfaguanidine (G1) and cisplatin (G2). 1H NMR results show that G1 and G2 can be selectively combined in two different cavities of C1. The same host-guest complex could be obtained, whether G1 and G2 were added at the same time or in a stepwise fashion. This work laid a foundation for drug delivery and provided a new inspiration for concomitant drugs.
Xiuqing Huang , Qi Zhang . A Gourd-shaped Organometallic Coordination Cage: Synthesis and Selective Binding of Two Drug Molecules[J]. Acta Chimica Sinica, 2023 , 81(3) : 217 -221 . DOI: 10.6023/A22120511
| [1] | (a) Li M.; Jiang S.; Zhang Z.; Hao X.; Jiang X.; Yu H.; Wang P.; Xu B.; Wang M.; Tian W. CCS Chemistry 2020, 2, 337. |
| [1] | (b) Zhang Z.; Ma L.; Fang F.; Hou Y.; Lu C.; Mu C.; Zhang Y.; Liu H.; Gao K.; Wang M.; Zhang Z.; Li X.; Zhang M. JACS Au 2022, 2, 1479. |
| [1] | (c) Wang H.; Li Y.; Li N.; Filosa A.; Li X. Nat. Rev. Mater. 2020, 6, 145. |
| [1] | (d) Wang H.; Guo C.; Li X. CCS Chemistry 2022, 4, 785. |
| [1] | (e) Shi J.; Li Y.; Jiang X.; Yu H.; Li J.; Zhang H.; Trainer D. J.; Hla S. W.; Wang H.; Wang M.; Li X. J. Am. Chem. Soc. 2021, 143, 1224. |
| [1] | (f) Pan M.; Wu K.; Zhang J.; Su C. Coord. Chem. Rev. 2019, 378, 333. |
| [1] | (g) Mai H. D.; Tran N. M.; Yoo H. Coord. Chem. Rev. 2019, 387, 180. |
| [1] | (h) Li Y.; Wang H.; Li X. Chem. Sci. 2020, 11, 12249. |
| [1] | (i) Lee S.; Jeong H.; Nam D.; Lah M. S.; Choe W. Chem. Soc. Rev. 2021, 50, 528. |
| [2] | McMorran D. A.; Steel P. J. Angew. Chem. Int. Ed. 1998, 37, 3295. |
| [3] | (a) Wang J.; Young T. A.; Duarte F.; Lusby P. J. J. Am. Chem. Soc. 2020, 142, 17743. |
| [3] | (b) Spicer R. L.; Stergiou A. D.; Young T. A.; Duarte F.; Symes M. D.; Lusby P. J. J. Am. Chem. Soc. 2020, 142, 2134. |
| [4] | (a) Kaiser F.; Schmidt A.; Heydenreuter W.; Altmann P. J.; Casini A.; Sieber S. A.; Kühn F. E. Eur. J. Inorg. Chem. 2016, 2016, 5189. |
| [4] | (b) Lewis J. E. M.; Gavey E. L.; Cameron S. A.; Crowley J. D. Chem. Sci. 2012, 3, 778. |
| [4] | (c) Schmidt A.; Molano V.; Hollering M.; Pothig A.; Casini A.; Kuhn F. E. Chem 2016, 22, 2253. |
| [5] | (a) Sumida R.; Tanaka Y.; Niki K.; Sei Y.; Toyota S.; Yoshizawa M. Chem. Sci. 2021, 12, 9946. |
| [5] | (b) Yamashina M.; Tsutsui T.; Sei Y.; Akita M.; Yoshizawa M. Sci. Adv. 2019, 5, eaav3179. |
| [5] | (c) Niki K.; Tsutsui T.; Yamashina M.; Akita M.; Yoshizawa M. Angew. Chem. Int. Ed. 2020, 59, 10489. |
| [6] | Preston D.; White K. F.; Lewis J. E. M.; Vasdev R. A. S.; Abrahams B. F.; Crowley J. D. Chem 2017, 23, 10559. |
| [7] | (a) Lewis J. E. M.; Tarzia A.; White A. J. P.; Jelfs K. E. Chem. Sci. 2019, 11, 677. |
| [7] | (b) Yu H.; Li J.; Shan C.; Lu T.; Jiang X.; Shi J.; Wojtas L.; Zhang H.; Wang M. Angew. Chem. Int. Ed. 2021, 60, 26523. |
| [8] | Lisboa L. S.; Findlay J. A.; Wright L. J.; Hartinger C. G.; Crowley J. D. Angew. Chem. Int. Ed. 2020, 59, 11101. |
| [9] | (a) Preston D.; Barnsley J. E.; Gordon K. C.; Crowley J. D. J. Am. Chem. Soc. 2016, 138, 10578. |
| [9] | (b) Johnson A. M.; Moshe O.; Gamboa A. S.; Langloss B. W.; Limtiaco J. F.; Larive C. K.; Hooley R. J. Inorg. Chem. 2011, 50, 9430. |
| [9] | (c) Liu Y.; Liao S.; Dai W.; Bai Q.; Lu S.; Wang H.; Li X.; Zhang Z.; Wang P.; Lu W.; Zhang Q. Angew. Chem. Int. Ed. 2022, 61, e202217215. |
| [10] | Zhu R.; Regeni I.; Holstein J. J.; Dittrich B.; Simon M.; Prevost S.; Gradzielski M.; Clever G. H. Angew. Chem. Int. Ed. 2018, 57, 13652. |
| [11] | Yazaki K.; Akita M.; Prusty S.; Chand D. K.; Kikuchi T.; Sato H.; Yoshizawa M. Nat. Commun. 2017, 8, 15914. |
| [12] | Yamaguchi T.; Tashiro S.; Tominaga M.; Kawano M.; Ozeki T.; Fujita M. J. Am. Chem. Soc. 2004, 126, 10818. |
| [13] | Samantray S.; Krishnaswamy S.; Chand D. K. Nat. Commun. 2020, 11, 880. |
| [14] | Preston D.; Lewis J. E.; Crowley J. D. J. Am. Chem. Soc. 2017, 139, 2379. |
| [15] | Lisboa L. S.; Preston D.; McAdam C. J.; Wright L. J.; Hartinger C. G.; Crowley J. D. Angew. Chem. Int. Ed. 2022, 61, e202201700. |
| [16] | Lewis J. M. Angew. Chem. Int. Ed. 2022, 61, e202212392. |
| [17] | O'Connor, H. M.; Tipping, W. J.; Vallejo, J.; Nichol, G. S.; Faulds, K.; Graham, D.; Brechin, E. K.; Lusby, P. J. Inorg. Chem. 2022,10.1021/acs.inorgchem.2c00873. |
| [18] | (a) Liu Q. L.; Fang P. J.; Zhao Z. L.; Zhang H. Z.; Zhou C. H. Chin. J. Org. Chem. 2017, 37, 3146. (in Chinese) |
| [18] | (刘庆龙, 房鹏金, 赵志龙, 张慧珍, 周成合, 有机化学, 2017, 37, 3146). |
| [18] | (b) Zhang H. Z.; He S. C.; Peng Y. J.; Zhang H. J.; Gopala L.; Tangadanchu V. K. R.; Gan L. L.; Zhou C. H. Eur. J. Med. Chem. 2017, 136, 165. |
| [19] | Kelland L. Nat. Rev. Cancer. 2007, 7, 573. |
/
| 〈 |
|
〉 |