

Design, Synthesis and Photodynamic Therapy of a H2O2-Activatable Near Infrared Borondipyrromethene (BODIPY) Photosensitizer
Received date: 2022-12-04
Online published: 2023-03-09
Supported by
National Natural Science Foundation of China(21778036); National Natural Science Foundation of China(21877077)
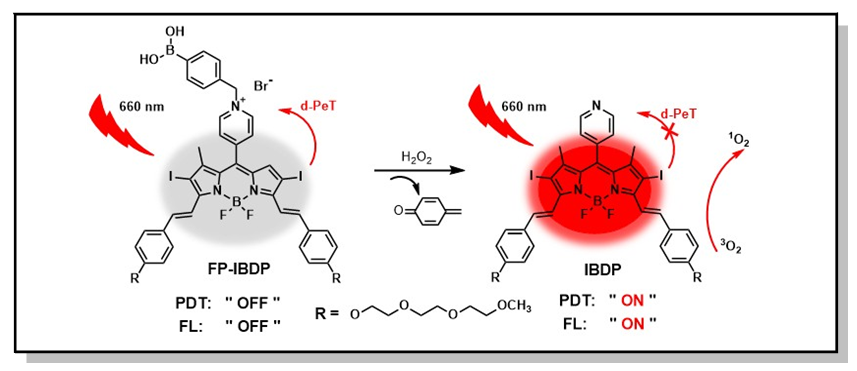
In this work, a borondipyrromethene (BODIPY)-based activatable photosensitizer FP-IBDP was designed and synthesized, which has absorption and emission wavelengths in the near infrared (NIR) region. The maximum absorption and emission of FP-IBDP are 681 nm and 740 nm in acetonitrile. The fluorescence quantum yield and singlet oxygen yield of FP-IBDP is 0.01 and 0.09, respectively. After activated by H2O2, FP-IBDP was transformed to photosensitizer IBDP, which has maximum absorption and emission peak at 661 nm and 701 nm in acetonitrile. Compared with FP-IBDP, the fluorescence quantum yield and singlet oxygen yield of IBDP are much increased, up to 0.11 and 0.48 respectively. It is demonstrated that FP-IBDP can respond H2O2 sensitively in cancer cells and has the ability to distinguish cancer cells from normal cells by a fluorescence enhancement way. Reactive oxygen species (ROS) detection indicated that FP-IBDP could be activated by the endogenous H2O2 in cancer cells and produce highly toxic singlet oxygen under 660 nm light excitation with dichlorodihydrofluorescein diacetate (DCFH-DA) as ROS indicator. Tetrazolium (MTT) assay indicated that FP-IBDP has good biocompatibility and low dark toxicity to both cancer cells and normal cells, while phototoxicity test and living/dead cell double staining experiment proved that FP-IBDP has higher phototoxicity to cancer cells than to normal cells. Besides, wounding healing assay confirmed its good ability to inhibit cancer cell proliferation. Fluorescence localization and lysosome disruption assays further indicated that FP-IBDP was specifically located in lysosomes of living cells and the produced singlet oxygen could induce cancer cell death in a lysosome disruption associated pathway. These features are expected to lay good foundation for the application of FP-IBDP in imaging guided photodynamic therapy under NIR excitation.
Xin Lv , Yi Wu , Boran Zhang , Wei Guo . Design, Synthesis and Photodynamic Therapy of a H2O2-Activatable Near Infrared Borondipyrromethene (BODIPY) Photosensitizer[J]. Acta Chimica Sinica, 2023 , 81(4) : 359 -370 . DOI: 10.6023/A22120487
| [1] | Li, X.; Lee, S.; Yoon, J. Chem. Soc. Rev. 2018, 47, 1174. |
| [2] | Luby, B.; Walsh, C.; Zheng, G. Angew. Chem. Int. Ed. 2019, 58, 2558. |
| [3] | Pham, T.; Nguyen, V.; Choi, Y.; Lee, S.; Yoon, J. Chem. Rev. 2021, 121, 13454. |
| [4] | Zhou, L.; Lv, F.; Liu, L.; Wang, S. Acc. Chem. Res. 2019, 52, 3211. |
| [5] | Yan, T.; Liu, Z.-H.; Song, X.-Y.; Zhang, S.-S. Acta Chim. Sinica 2020, 78, 657. (in Chinese) |
| [5] | (闫涛, 刘振华, 宋昕玥, 张书圣, 化学学报, 2020, 78, 657.) |
| [6] | Bu, Y.; Zhu, X.; Wang, H.; Zhang, J.; Wang, L.; Yu, Z.; Tian, Y.; Zhou, H.; Xie, Y. Anal. Chem. 2021, 93, 12059. |
| [7] | Chen, D.; Wang, Z.; Dai, H.; Lv, X.-Y.; Ma, Q.; Yang, D.; Shao, J.; Xu, Z.; Dong, X. Small Methods 2020, 2000013. |
| [8] | Chen, L.; Yang, Y.; Zhang, P.; Wang, S.; Xu, J. F.; Zhang, X. ACS Appl. Bio Mater. 2019, 2, 2920. |
| [9] | Feng, L.; Betzer, O.; Tao, D.; Sadan, T.; Popovtzer, R.; Liu, Z. CCS Chem. 2019, 1, 239. |
| [10] | Triesscheijn, M.; Baas, P.; Schellens, J. M.; Stewart, F. A. Oncology 2006, 11, 1034. |
| [11] | Gross, S.; Gilead, A.; Scherz, A.; Neeman, M.; Salomon, Y. Nat. Med. 2003, 9, 1327. |
| [12] | Master, A.; Livingston, M.; Sen, A. J. Control. Release 2013, 168, 88. |
| [13] | Cao, H.; Wang, L.; Yang, Y.; Li, J.; Qi, Y.; Li, Y.; Wang, H.; Li, J.-B. Angew. Chem. Int. Ed. 2018, 57, 7759. |
| [14] | Wang, S.-B.; Han, K.; Lei, Q.; Zhu, J.-Y.; Zhang, X.-Z. ACS Nano 2015, 9, 10268. |
| [15] | Zhai, W.-H.; Zhang, Y.-K.; Liu, M.; Zhang, H.; Zhang, J.-P.; Li, C.-H. Angew. Chem. Int. Ed. 2019, 58, 16601. |
| [16] | Tian, J.; Ding, L.; Xu, H.; Shen, Z.; Ju, H.; Jia, L.; Bao, L.; Yu, J. J. Am. Chem. Soc. 2013, 135, 18850. |
| [17] | Ling, D.; Park, W.; Park, S. j.; Lu, Y.; Kim, K.; Hackett, M.; Kim, B.; Yim, H.; Jeon, Y.; Na, K.; Hyeon, T. J. Am. Chem. Soc. 2014, 136, 5647. |
| [18] | Tian, J.; Ding, L.; Ju, H.; Yang, Y.; Li, X.; Shen, Z.; Zhu, Z.; Yu, J.; Yang, C. Angew. Chem. Int. Ed. 2014, 53, 9544. |
| [19] | Zhang, Y.; Li, X.; Huang, L.; Kim, H.; An, J.; Lan, M.; Cao, Q.; Kim, J. Chem. Commun. 2020, 56, 10317. |
| [20] | Xu, F.; Li, H.; Yao, Q.; Ge, H.; Fan, J.; Sun, W.; Wang, J.; Peng, X. Chem. Sci. 2019, 10, 10586. |
| [21] | Guo, Z.; Park, S.; Yoon, J.; Shin, I. Chem. Soc. Rev. 2014, 43, 16. |
| [22] | Chen, X.; Lee, D.; Yu, S.; Kim, G.; Lee, S.; Cho, Y.; Jeong, H.; Nam, K.; Yoon, J. Biomaterials 2017, 122, 130. |
| [23] | Tian, R.; Sun, W.; Li, M.; Long, S.; Li, M.; Fan, J.; Guo, L.; Peng, X. Chem. Sci. 2019, 10, 10106. |
| [24] | Awuah, S.; You, Y. RSC Adv. 2012, 2, 11169. |
| [25] | Bessette, A.; Hanan, G. Chem. Soc. Rev. 2014, 43, 3342. |
| [26] | Liu, B.-D.; Wang, C.-J.; Qian, Y. Acta Chim. Sinica 2022, 80, 1071. (in Chinese) |
| [26] | (刘巴蒂, 王承俊, 钱鹰, 化学学报, 2022, 80, 1071.) |
| [27] | Yogo, T.; Urano, Y.; Ishitsuka, Y.; Maniwa F.; Nagano, T. J. Am. Chem. Soc. 2005, 127, 12162. |
| [28] | Atilgan, S.; Ekmekci, Z.; Dogan, A. L.; Guc, D.; Akkaya, E. Chem. Commun. 2006, 4398. |
| [29] | Awuah, S. G.; Polreis, J.; Biradar, V.; You, Y. Org. Lett. 2011, 13, 3884. |
| [30] | Batat, P.; Cantuel, M.; Jonusauskas, G.; Scarpantonio, L.; Palma, A.; O’Shea, D.; McClenaghan, N. J. Phys. Chem. A 2011, 115, 14034. |
| [31] | Nguyen, V.; Yim, Y.; Kim, S.; Ryu, B.; Swamy, K.; Kim, G.; Kwon, N.; Kim, C.; Park, S.; Yoon, J. Angew. Chem. Int. Ed. 2020, 59, 8957. |
| [32] | Dong, Y.; Dick, B.; Zhao, J. Z. Org. Lett. 2020, 22, 5535. |
| [33] | Raza, M.; Gautam, S.; Howlader, P.; Bhattacharyya, A.; Kondaiah, P.; Chakravarty, A. Inorg. Chem. 2018, 57, 14374. |
| [34] | Li, M.; Tian, R.; Fan, J.; Du, J.; Long, S.; Peng, X. Dyes and Pigments 2017, 147, 99. |
| [35] | Liu, Y.; Xu, C.; Teng, L.; Liu, H.; Ren, T.; Xu, S.; Lou, X.; Guo, H.; Yuan, L.; Zhang, X. Chem. Commun. 2020, 56, 1956. |
| [36] | Teng, K.; Niu, L.; Kang, Y.; Yang, Q. Chem. Sci. 2020, 11, 9703. |
| [37] | Lv, X.; Han, T.; Wu, Y.; Zhang, B.; Guo, W. Chem. Commun. 2021, 57, 9744. |
| [38] | Yuan, B.; Wang, H.; Xu, J.; Zhang, X. ACS Appl. Mater. Interfaces 2020, 12, 26982. |
| [39] | Zeng, Q.; Zhang, R.; Zhang, T.; Xing, D. Biomaterials 2019, 207, 39. |
| [40] | Liu, H.; Hu, X.; Li, K.; Liu, Y.; Rong, Q.; Zhu, L.; Yuan, L.; Qu, F.; Zhang, X.; Tan, W. Chem. Sci. 2017, 8, 7689. |
| [41] | Chen, H.; Tian, J.; He, W.; Guo, Z. J. Am. Chem. Soc. 2015, 137, 1539. |
| [42] | Abouelmagd, S. A.; Hyun, H.; Yeo, Y. Expert Opin. Drug Del. 2014, 11, 1601. |
| [43] | Hamblin, M. R.; Miller, J. L.; Rizvi, I.; Ortel, B.; Maytin, E. V.; Hasan, T. Cancer Res. 2001, 61, 7155. |
| [44] | Sahoo, S. K.; Sawa, T.; Fang, J.; Tanaka, S.; Miyamoto, Y.; Akaike, T.; Maeda, H. Bioconjugate Chem. 2002, 13, 1031. |
| [45] | Rapozzi, V.; Zacchigna, M.; Biffi, S.; Garrovo, C.; Cateni, F.; Stebel, M.; Zorzet, S.; Bonora, G. M.; Drioli, S.; Xodo, L. Cancer Biol. Ther. 2010, 10, 471. |
| [46] | Narayanaswamy, N.; Narra, S.; Nair, R.; Saini, D.; Kondaiah, P.; Govindaraju, T. Chem. Sci. 2016, 7, 2832. |
| [47] | Reja, S.; Gupta, M.; Gupta, N.; Bhalla, V.; Ohri, P.; Kaur, G.; Kumar, M. Chem. Commun. 2017, 53, 3701. |
| [48] | Guicciardi, M.; Leist, M.; Gores, G. Oncogene 2004, 23, 2881. |
| [49] | Kroemer, G.; Jaattela, M. Nat. Rev. Cancer 2005, 5, 886. |
| [50] | Chen, H.; Xiao, L.; Anraku, Y.; Mi, P.; Liu, X.; Cabral, H.; Inoue, A.; Nomoto, T.; Kishimura, A.; Nishiyama, N.; Kataoka, K. J. Am. Chem. Soc. 2014, 136, 157. |
/
| 〈 |
|
〉 |