

Study on Construction and Performance of Immobilized Enzyme Reactors by Carboxyl-functionalized Fe3O4
Received date: 2023-01-18
Online published: 2023-03-28
Supported by
Scientific Research Program Funded by Shaanxi Provincial Education Department(16JK1827); Scientific Research Program Funded by Shaanxi Provincial Education Department(22JK0605); Natural Science Basic Research Program of Shaanxi(2023-JC-QN-0173)
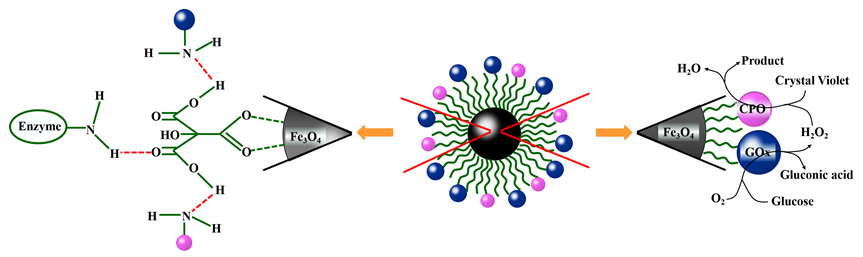
The interface connection between carrier and enzyme can be improved by functionalizing the carrier of immobilized enzyme. This facilitates the formation of highly ordered two-dimensional arrangement of enzyme molecules on the surface of the carrier, thus improving the catalytic activity and operational stability of the enzymes. The surface of Fe3O4 modified with citric acid (CA-Fe3O4) is rich in carboxyl groups, which can be used as an excellent carrier due to its magnetic and easy separation characteristics. In this work, the adsorption method was more suitable for constructing enzyme reactors on the carrier of CA-Fe3O4, compared with covalent method. The CPO@CA-Fe3O4 reactor was constructed to immobilize chloroperoxidase (CPO) and the cascade enzyme reactor of GOx&CPO@CA-Fe3O4 was constructed by co-immobilization of CPO and glucose oxidase (GOx). In catalytic reaction, H2O2 was used as oxidant for CPO@CA-Fe3O4, while for GOx&CPO@CA-Fe3O4, the in-situ formation of H2O2 was caused by the oxidation of β-D-glucose by GOx. When the molar ratio of CPO to GOx was 3∶4 for immobilization, the effect of the cascade reaction can reach the best. When the enzyme reactors were applied to catalyze the oxidation of the decolorization of crystal violet, both enzyme reactors showed good catalytic activity, affinity and specificity for substrate. The thermal stabilities of the immobilized enzyme reactors were significantly improved. Both the enzyme reactors retained more than 55% of the activity at 70 ℃ for 1 h and more than 60% of the activity at 50 ℃ for 8 h, while the free enzyme was almost completely inactivated under the same reaction conditions. In contrast with CPO@CA-Fe3O4, the GOx&CPO@CA-Fe3O4 reactor showed better catalytic performance due to the in-situ generation of H2O2 in the cascade reaction. And this advantage was particularly reflected in the application of thermal stability and actual water samples. The important application potential has been shown about the immobilization of CPO enzyme reactors on CA-Fe3O4 carrier as a catalyst.
Fengqin Gao , Yang Liu , Yinli Zhang , Yucheng Jiang . Study on Construction and Performance of Immobilized Enzyme Reactors by Carboxyl-functionalized Fe3O4[J]. Acta Chimica Sinica, 2023 , 81(4) : 338 -344 . DOI: 10.6023/A23010015
| [1] | Rana, H.; Sharma, A.; Dutta, S.; Goswami, S. J. Polym. Environ. 2022, 30, 4936. |
| [2] | Wu, H.; Mu, W.-M. Curr. Opin. Food Sci. 2022, 47, 100909. |
| [3] | Gao, X.; Pan, H.-B.; He, Z.-X.; Yang, K.; Qiao, C.-F.; Liu, Y.-L.; Zhou, C.-S. Acta Chim. Sinica 2021, 79, 1502. (in Chinese) |
| [3] | (高霞, 潘会宾, 贺曾贤, 杨柯, 乔成芳, 刘永亮, 周春生, 化学学报, 2021, 79, 1502.) |
| [4] | Lyu, F.-J.; Zhang, Y. F.; Zare, R. N.; Ge, J.; Liu, Z. Nano Lett. 2014, 14, 5761. |
| [5] | Li, S.-F.; Chen, Y.; Wang, Y.-S.; Mo, H.-L.; Zang, S.-Q. Sci. China: Chem. 2022, 65, 1122. |
| [6] | Xiong, Y.; Tsitkov, S.; Hess, H.; Gang, O.; Zhang, Y.-F. ACS Nano 2022, 16, 10383. |
| [7] | Wu, X. L.; Ge, J.; Yang, C.; Hou, M.; Liu, Z. Chem. Commun. 2015, 51, 13408. |
| [8] | Qiao, J.; Zhang, X.-Y.; Qi, L. ACS Appl. Bio Mater. 2022, 5, 4264. |
| [9] | Matera, A.; Dulak, K.; Sordon, S.; Wa?niewski, K.; Huszcza, E.; Pop?oński, J. Appl. Microbiol. Biotechnol. 2022, 106, 7763. |
| [10] | Xia, H.; Li, N.; Huang, W.-Q.; Song, Y.; Jiang, Y.-B. ACS Appl. Mater. Interfaces 2021, 13, 22240. |
| [11] | Wang, Y.; Zhang, X.; Wei, Z.-H.; Jiao, Y.-J.; An, D. Y.; Huang, Y. P.; Liu, Z.-S.; Yan, C. J. Chromatogr. A 2022, 1666, 462848. |
| [12] | Tvorynska, S.; Barek, J.; Josypcuk, B. Bioelectrochemistry 2022, 148, 108223. |
| [13] | Bahadur, A.; Saeed, A.; Shoaib, M.; Iqbal, S.; Bashir, M. I.; Waqas, M.; Hussain, M. N.; Abbas, N. Mater. Chem. Phys. 2017, 198, 229. |
| [14] | Mukhortova, Y. R.; Pryadko, A. S.; Chernozem, R. V.; Pariy, I. O.; Akoulina, E. A.; Demianova, I. V.; Zharkova, I. I.; Ivanov, Y. F.; Wagner, D. V.; Bonartsev, A. P.; Surmenev, R. A.; Surmeneva, M. A. Nano-Struct. Nano-Objects 2022, 29, 100843. |
| [15] | Zhao, Y.; Yuan, L.; Bai, X.-L.; Jiang, X.-X.; Zhang, Y.; Fang, Q.; Zhang, Q.; Liao, X. J. Sep. Sci. 2022, 45, 3635. |
| [16] | Sarno, M.; Iuliano, M.; Polichetti, M.; Ciambelli, P. Process Biochem. 2017, 56, 98. |
| [17] | Lv, C.-H.; Yang, X.-W.; Wang, Z.-K.; Ying, M.; Han, Q.-G.; Li, S.-F. Nanomaterials 2021, 11, 3086. |
| [18] | Zhang, Y.; He, S.; Simpson, B. K. Curr. Opin. Food Sci. 2018, 19, 30. |
| [19] | Verma, K.; Saha, G.; Kundu, L. M.; Dubey, V. K. Int. J. Biol. Macromol. 2019, 121, 1011. |
| [20] | Cheng, H.-P; Hu, M.-C.; Zhai, Q.-G.; Li, S.-N.; Jiang, Y.-C. Chem. Eng. J. 2018, 347, 703. |
| [21] | Jin, X.-Y.; Li, S.-S.; Long, N.-B.; Zhang, R.-F. Appl. Biochem. Biotechnol. 2018, 184, 1009. |
| [22] | Lu, J.; Cheng, L.; Wang, Y.; Ding, Y.; Hu, M.-C.; Li, S.-N.; Zhai, Q.-G.; Jiang, Y.-C. Mater. Des. 2017, 129, 219. |
| [23] | Wang, S.-J.; Ding, Y.; Chen, R.; Hu, M.-C.; Li, S.-N.; Zhai, Q.-G.; Jiang, Y.-C. Chem. Eng. Res. Des. 2018, 134, 52. |
| [24] | Cui, R.; Bai, C.-H.; Jiang, Y.-C.; Hu, M.-C.; Li, S.-N.; Zhai, Q.-G. Chem. Eng. J. 2015, 259, 640. |
| [25] | Nikazar, M.; Alizadeh, M.; Lalavi, R.; Rostami, M. H. J. Environ. Health Sci. 2014, 12, 21. |
| [26] | Wang, G.-S.; Geng, J.-H.; Guo, T.-L.; Qi, X.-W.; Yu, R.-T.; Zhang, Z.-X.; Wang, Y.-M.; Wang, Z.-H.; Li, Z.-Q.; Li, P.; Li, D.; Chang, G.-Q. Ceram. Int. 2022, 48, 29031. |
| [27] | Bahadur, A.; Saeed, A.; Shoaib, M.; Iqbal, S.; Bashir, M. I.; Waqas, M.; Hussain, M. N.; Abbas, N. Mater. Chem. Phys. 2017, 198, 229. |
| [28] | Jin, R.-X.; Li, C.-N.; Zhi, L.-F.; Jiang, Y.-C.; Hu, M.-C.; Li, S.-N.; Zhai, Q.-G. Carbohydr. Res. 2013, 370, 72. |
| [29] | Zhang, J.-F.; Li, Y.-J.; Zhang, Y.-X.; Wu, Y.-H.; Ju, J.-L.; He, W.; Li, C.-C. Nano 2022, 17, 2250062. |
| [30] | Li, C.-F.; Jiang, S.-H.; Zhao, X.-Y.; Liang, H. Molecules 2017, 22, 179. |
| [31] | Zhang, J.; Wang, Z.-J.; He, C.; Liu, X.-L.; Zhao, W.-F.; Sun, S.-D.; Zhao, C.-S. ACS Omega 2019, 4, 2853. |
| [32] | Zhao, R.-N.; Hu, M.-C.; Li, S.-N.; Zhai, Q.-G.; Jiang, Y.-C. Acta Chim. Sinica 2017, 75, 293. (in Chinese) |
| [32] | (赵睿南, 胡满成, 李淑妮, 翟全国, 蒋育澄, 化学学报, 2017, 75, 293.) |
/
| 〈 |
|
〉 |