

Sovlent Influence on the Femtosecond Transient Absorption Spectra of Tetraphenylporphyrin Manganese(III) Chloride
Received date: 2023-01-27
Online published: 2023-04-06
Supported by
National Natural Science Foundation of China(21671068); 2018 local Development Research Center Project of Yichun Univeristy(DF2018021); Science and Technology Research Project of Education Comission of Jiangxi Province(GJJ2201723); Science and Technology Research Project of Education Comission of Jiangxi Province(GJJ2201724); Science and Technology Research Project of Health Commission of Jiangxi Province(202212701); Open Fund of Key Laboratory of Green Chemical Technology of Fujian Province University(WYKF-GCT2022-1)
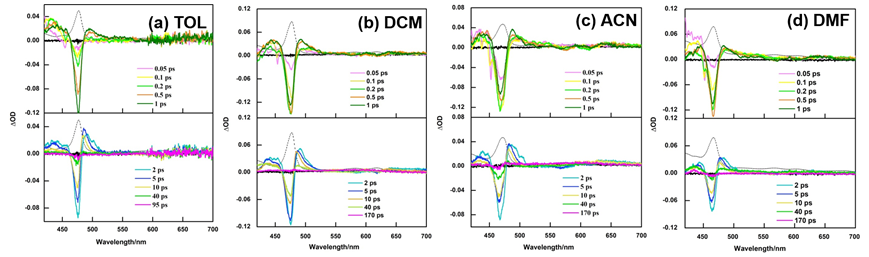
The photophysical properties of tetraphenylporphyrin manganese(III) chloride MnIII(TPP)Cl in four solvents of toluene (TOL), dichloromethane (DCM), acetonitrile (ACN) and N,N-dimethylformamide (DMF) were investigated through the steady-state and the femtosecond transient absorption spectroscopy. The MnIII(TPP)Cl molecule is populated to the lifetimes of the second excited singlet state (5S2) with pump pulse at 400 nm. Broad band white light continuum in the visible region from 420 nm to 700 nm is used in the probe. With a detail analysis of the measured transient spectra, three decay time constants τ1, τ2 and τ3 corresponding to the lifetime of the second excited singlet state (5S2), the lifetime of the first excited singlet state (5S1) and the first excited triplet state (5T1) are obtained in the four solvents. The results show that the steady-state B-band and Q-band absorption and the bleaching signal of the femtosecond transient absorption exhibit blue-shift. With the increase of the concentration of MnIII(TPP)Cl in DCM, the lifetimes of the second excited singlet state (5S2) had no significant change. And the lifetimes of the first excited singlet state (5S1) and the first excited triplet state (5T1) shortened. In these four solvents, its 5S2 lifetime in nonpolar solvent TOL was the longest and the 5S1 lifetime, 5T1 lifetime were the shortest. This is in accord with the largest 5S2→5S1 energy gap and the least 5S1→5S0 energy gap in TOL. From weak polar solvent DCM to the more polar solvent DMF, the 5S1 lifetime and 5T1 lifetime shortened. With the solvent polarity increasing from nonpolar solvent TOL to the more polar solvent ACN, the 5S2 lifetime shortened. While its 5S2 lifetime becomes longer in polar solvent DMF. This is mainly caused by the axial ligation of nitrogen atom of coordinating solvent DMF with 5S2 of MnIII(TPP)Cl. The structure of MnIII(TPP)Cl changed from five-coordinate to six-coordinate. As a result, the longer 5S2 lifetime in DMF should be due to the combined influence of the polarity of solvent and the axial ligation and the latter plays the main part.
Wanhong Li , Mingyue Yu , Lili Wang , Dehuang Zhu , Suhong Peng , Hui Wang , Haiyang Liu . Sovlent Influence on the Femtosecond Transient Absorption Spectra of Tetraphenylporphyrin Manganese(III) Chloride[J]. Acta Chimica Sinica, 2023 , 81(4) : 345 -350 . DOI: 10.6023/A23010017
| [1] | Liu, H. Y.; Mahmood, M. H. R.; Qiu, S. X.; Chang, C. K. Coord. Chem. Rev. 2013, 257, 1306. |
| [2] | Peng, S. H.; Zhou, R.; Zou, H. B. Chinese J. Org. Chem. 2019, 39, 3384. (in Chinese) |
| [2] | (彭素红, 周蓉, 邹怀波, 有机化学, 2019, 39, 3384.) |
| [3] | Mauzerall, D. In the porphyrins handbook, Vol. 5, Ed.: Dolphin, D., Academic Press, New York, 1978, p. 29. |
| [4] | Weschler, C. J.; Hoffman, B. M.; Basolam F. J. Am. Chem. Soc. 1975, 97, 5278. |
| [5] | Hoffman, B. M.; Weschler, C. J.; Basola, F. J. Am. Chem. Soc. 1976, 98, 473. |
| [6] | Harriman, A. J. Chem. Soc., Faraday Trans 1 1981, 77, 369. |
| [7] | Irvine, M. P.; Harrison, R. J.; Strahand, M. A.; Beddard, G. S. Ber. Bunsen-Ges. Phys. Chem. 1985, 89, 226. |
| [8] | Yan, X. W.; Kirmaier, C.; Holten, D. Inorg. Chem. 1986, 25, 4774. |
| [9] | Kim, Y.; Choi, J. R.; Yoon, M.; Furube, A.; Asahi, T.; Masuhara, H. J. Phys. Chem. B 2001, 105, 8513. |
| [10] | Wang, L. L.; Peng, S. H.; Wang, H.; Ji, L. N.; Liu, H. Y. Phys. Chem. Chem. Phys. 2018, 20, 20141. |
| [11] | Fan, M. G.; Tong, Z. H. Molecular Photochemistry, Science Press, Beijing, 2013, p. 25. (in Chinese) |
| [11] | (樊美公, 佟振合, 分子光化学, 科学出版社, 北京, 2013, p. 25.) |
| [12] | Wang, L. L.; Wang, H.; Chen, F.; Liang, Z. H.; Liu, C. F.; Li, Y.; Wang, W. Q.; Peng, S. H.; Wang, X.; Ying, X.; Ji, L. N.; Liu, H. Y. J. Phys. Chem. C 2017, 121, 12350. |
| [13] | Hurjui, I; Ivan, L. M.; Dorohoi, D. O. Spectrochim, Acta, Part A 2013, 102, 219. |
| [14] | Yu, H. Z.; Baskin, J. S.; Zewail, A. H. J. Phys. Chem. A 2002, 106, 9845. |
| [15] | Baskin, J. S.; Yu, H. Z.; Zewail, A. H. J. Phys. Chem. A 2002, 106, 9837. |
| [16] | Br?m, O.; Consani, C.; Cannizzo, A.; Chergui, M. J. Phys. Chem. B 2011, 115, 13723. |
| [17] | Do?an, N.; Dumano?ullar?, F. M.; Hayval?, M.; Y?lmaz, H.; Kürüm, U.; Yaglioglu, H. G.; Elmali, A. Chem. Phys. Lett. 2011, 508, 265. |
| [18] | Velate, S.; Liu, X.; Steer, R. P. Chem. Phys. Lett. 2006, 427, 295. |
| [19] | Alder, A. D.; Longo, F. R.; Finrelli, J. D. J. Inorg. Nucl. Chem. 1970, 32, 2443. |
| [20] | Foggi, P.; Bussotti, L.; Neuwahl, F. V. R. Int. J. Photoenergy. 2001, 3, 103. |
/
| 〈 |
|
〉 |