

A High-entropy Phosphate Cathode Host towards High-stability Lithium-sulfur Batteries
Received date: 2023-02-02
Online published: 2023-04-06
Supported by
National Natural Science Foundation of China(52072256)
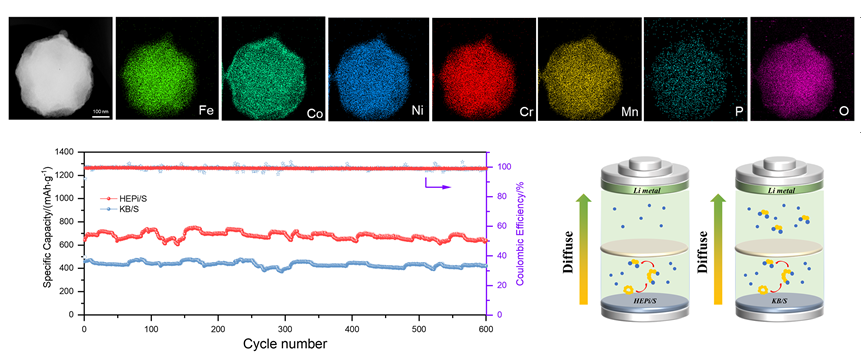
With the rapid development of portable electronic devices, electric vehicles and large-scale energy storage, higher energy density energy storage devices are needed to replace traditional lithium-ion batteries. Lithium-sulfur batteries (LSBs) are expected to be the next generation of high-specific capacity secondary batteries due to the high energy density (2600 Wh•kg-1) and low cost ($ 150 ton-1). However, LSBs also face serious problems, including poor rate performance and short cycle life. These problems are rooted in the insulating nature of sulfur, the shuttle effect of the intermediate phase lithium polysulfide (LiPSs), and sluggish reaction of sulfur redox reaction. Severe shuttle effects can cause LiPSs to diffuse into the lithium cathode and deposit on the Li metal surface, leading to the loss of active material; the insulating properties of sulfur and the reaction barrier also greatly limit the multiplier performance of lithium-sulfur batteries. The use of electrochemical catalysts to accelerate the conversion between LiPSs and lithium sulfide is currently an effective solution. Among them, high-entropy compounds have attracted a lot of attention. High entropy compounds are composed of multiple metallic elements uniformly distributed in the solid solution, and the catalytic activity and stability of these compounds are significantly enhanced due to the synergistic effect and high entropic stability. However, currently developed multi-elemental and high-entropy compounds are limited to single anion species such as oxides, carbides and sulfides, which have relatively simple molecular structures. Although the choice of combinatorial elements has been broadened by introducing the concept of high entropy, there is still a vast scope for development by extending the synthetic capabilities to more complex systems such as polyanionic materials. In this study, a high-entropy metal phosphate (HEPi) catalyst is obtained by one-step spray pyrolysis and applied to the sulfur cathode host. The rich porous structure of HEPi not only facilitates the encapsulation and domain-limiting of sulfur and ensures the sufficient infiltration of electrolyte, but also enhances the chemisorption of polysulfides and accelerates the rapid conversion of polysulfides, thus suppressing the shuttle effect of polysulfides and improving the utilization of sulfur. The results show that the assembled cell can obtain a high discharge capacity of 1477.3 mAh•g-1 at a current density of 0.1 C. The capacity can be maintained at 782 mAh•g-1 even at a high current density of 2 C. This work offers a prospect for the application of high-entropy materials in lithium-sulfur batteries.
Zhenxin Zhao , Yikun Yao , Jiajun Chen , Rong Niu , Xiaomin Wang . A High-entropy Phosphate Cathode Host towards High-stability Lithium-sulfur Batteries[J]. Acta Chimica Sinica, 2023 , 81(5) : 496 -501 . DOI: 10.6023/A23020020
| [1] | Wang, P.; Xi, B.; Huang, M.; Chen, W.; Feng, J.; Xiong, S. Adv. Energy Mater. 2021, 11, 2002893. |
| [2] | Liu, Y. T.; Liu, S.; Li, G. R.; Gao, X. P. Adv. Mater. 2020, 33, e2003955. |
| [3] | He, Y.; Zhao, Y.; Zhang, Y.; He, Z.; Liu, G.; Li, J.; Liang, C.; Li, Q. Energy Storage Materials 2022, 47, 434. |
| [4] | Li, X.; Zhang, Y.; Wang, S.; Liu, Y.; Ding, Y.; He, G.; Zhang, N.; Yu, G. Nano Lett. 2020, 20, 701. |
| [5] | Guo, W.; Han, Q.; Jiao, J.; Wu, W.; Zhu, X.; Chen, Z.; Zhao, Y. Angew. Chem. Int. Ed. 2021, 60, 7267. |
| [6] | Liu, T.; Hu, H.; Ding, X.; Yuan, H.; Jin, C.; Nai, J.; Liu, Y.; Wang, Y.; Wan, Y.; Tao, X. Energy Storage Mater. 2020, 30, 346. |
| [7] | Liu, H.; Jia, G.; Zhu, S.; Sheng, J.; Zhang, Z.; Li, Y. Acta Chim. Sinica 2022, 80, 89. (in Chinese) |
| [7] | 刘汉鼎, 贾国栋, 朱胜, 盛建, 张则尧, 李彦, 化学学报, 2022, 80, 89.) |
| [8] | Geng, X.; Lin, R.; Gu, X.; Su, Z.; Lai, C. Chinese J. Chem. 2021, 39, 1523. |
| [9] | Zhao, Z.; Yi, Z.; Li, H.; Pathak, R.; Yang, Z.; Wang, X.; Qiao, Q. Nano Energy 2021, 81, 105621. |
| [10] | Zhao, Z.; Duan, Y.; Chen, F.; Tian, Z.; Pathak, R.; Elam, J.; Yi, Z.; Wang, Y.; Wang, X. Chem. Eng. J. 2022, 450, 138310. |
| [11] | Feng, S.; Fu, Z. H.; Chen, X.; Zhang, Q. InfoMat 2022, 4, e12304. |
| [12] | Abdelhafiz, A.; Wang, B.; Harutyunyan, A.; Li, J. Adv. Energy Mater. 2022, 12, 2200742. |
| [13] | Xie, P.; Yao, Y.; Huang, Z.; Liu, Z.; Zhang, J.; Li, T.; Wang, G.; Shahbazian-Yassar, R.; Hu, L.; Wang, C. Nat. Commun. 2019, 10, 4011. |
| [14] | Wang, Z.; Zhang, X.; Wu, X.; Pan, Y.; Li, H.; Han, Y.; Xu, G.; Chi, J.; Lai, J.; Wang, L. Chem. Eng. J. 2022, 437, 135375. |
| [15] | Yang, J.; Guo, D.; Zhao, S.; Lin, Y.; Yang, R.; Xu, D.; Shi, N.; Zhang, X.; Lu, L.; Lan, Y. Q.; Bao, J.; Han, M. Small 2019, 15, e1804546. |
| [16] | Zhao, Z.; Yi, Z.; Li, H.; Pathak, R.; Cheng, X.; Zhou, J.; Wang, X.; Qiao, Q. Nanoscale 2021, 13, 14777. |
| [17] | Shen, Z.; Zhou, Q.; Yu, H.; Tian, J.; Shi, M.; Hu, C.; Zhang, H. Chinese J. Chem. 2021, 39, 1138. |
| [18] | Xia, G.; Ye, J.; Zheng, Z.; Li, X.; Chen, C.; Hu, C. Carbon 2021, 172, 96. |
| [19] | Chen, Y.; Zhang, W.; Zhou, D.; Tian, H.; Su, D.; Wang, C.; Stockdale, D.; Kang, F.; Li, B.; Wang, G. ACS Nano 2019, 13, 4731. |
| [20] | Zhang, K.; Zhao, Z.; Wang, X. J. Alloy. Compd. 2022, 906, 164376. |
| [21] | Cui, M.; Yang, C.; Li, B.; Dong, Q.; Wu, M.; Hwang, S.; Xie, H.; Wang, X.; Wang, G.; Hu, L. Adv. Energy Mater. 2020, 11, 2002887. |
| [22] | Qiao, H.; Wang, X.; Dong, Q.; Zheng, H.; Chen, G.; Hong, M.; Yang, C.-P.; Wu, M.; He, K.; Hu, L. Nano Energy 2021, 86, 106029. |
| [23] | Li, Y.; Wu, H.; Wu, D.; Wei, H.; Guo, Y.; Chen, H.; Li, Z.; Wang, L.; Xiong, C.; Meng, Q.; Liu, H.; Chan, C. Adv. Sci. (Weinh) 2022, 9, e2200840. |
| [24] | Zhang, J.; You, C.; Lin, H.; Wang, J. Energy Environ. Mater. 2022, 5, 731. |
| [25] | Hu, Z.; Yang, L.; Wang, X.; Wu, Q.; Chen, G.; Cheng, X.; Jiao, L.; He, J. Acta Chim. Sinica 2022, 80, 896. (in Chinese) |
| [25] | 胡征, 杨立军, 王喜章, 吴强, 陈光海, 程雪怡, 焦柳, 何家伟, 化学学报, 2022, 80, 896.) |
| [26] | Yao, W.; Tian, C.; Yang, C.; Xu, J.; Meng, Y.; Manke, I.; Chen, N.; Wu, Z.; Zhan, L.; Wang, Y.; Chen, R. Adv. Mater. 2022, 34, e2106370. |
| [27] | Wang, X.; Zhao, Z.; Cheng, X.; Chen, F. Acta Chim. Sinica 2021, 79, 941. (in Chinese) |
| [27] | 王晓敏, 赵振新, 程晓琴, 陈锋, 化学学报, 2021, 79, 941.) |
| [28] | Jiang, B.; Tian, D.; Qiu, Y.; Song, X.; Zhang, Y.; Sun, X.; Huang, H.; Zhao, C.; Guo, Z.; Fan, L.; Zhang, N. Nanomicro Lett. 2021, 14, 40. |
| [29] | Zhao, Z.; Yi, Z.; Duan, Y.; Pathak, R.; Cheng, X.; Wang, Y.; Elam, J.; Wang, X. Chem. Eng. J. 2023, 463, 142397. |
| [30] | Li, M.; Liu, W.; Luo, D.; Chen, Z.; Amine, K.; Lu, J. ACS Energy Lett. 2022, 7, 577. |
/
| 〈 |
|
〉 |