

Synthesis of New Sulfur-free and Phosphorus-free Ether-ester and Study on Its Properties As Ashless Friction Modifier
Received date: 2023-03-30
Online published: 2023-05-09
Supported by
Yunnan Ten Thousand Talents Plan Young & Elite Talents Project(YNWR-QNBJ-2018-067); Yunnan Ten Thousand Talents Plan Young & Elite Talents Project(YNWR-QNBJ-2020-002); LingChuang Research Project of China National Nuclear Corporation and Strategic Pilot Science and Technology Special Project of the Chinese Academy of Sciences (Class A) “Key Technologies and Demonstration of Transformative Clean Energy”(XDA21021203)
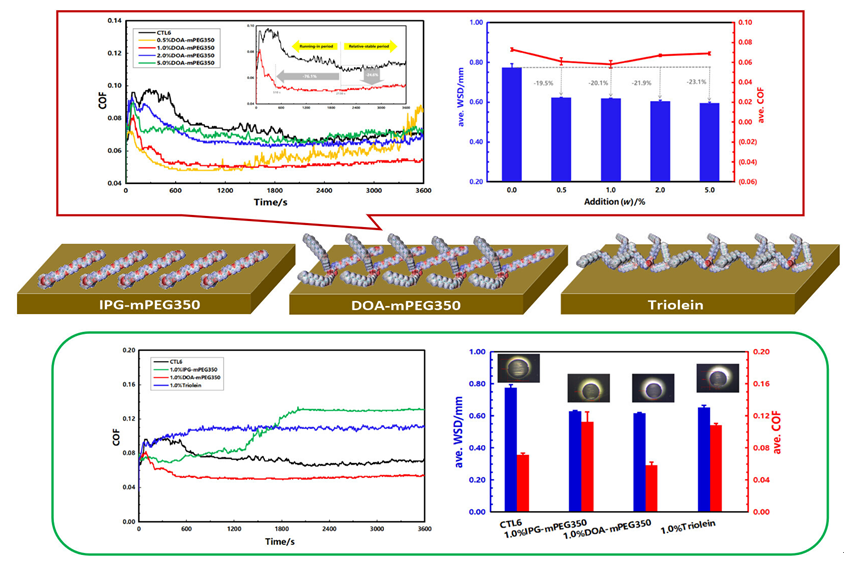
With the upgrading of mechanical equipment and the implementation of the “double-carbon” policy, the demand for high-quality lubricants with longer drain period, more clean and environmental friendly is increasing day by day. The study on new additives and base oils, as well as the compatibility law between the two has always run through the development of high-performance lubricants. As an important additive to improve the durability of lubricating protection and fuel economy, the design and development of friction modifier is the forefront of the development of lubrication technologies. Based on these, a new sulfur-free and phosphorus-free ether-ester DOA-mPEG350 was designed and synthesized, and characterized by nuclear magnetic resonance (NMR), high resolution mass spectrometer (HR-MS), Fourier transform infrared (FT-IR) and gel permeation chromatography (GPC). Meanwhile, the thermal stability of DOA-mPEG350 and its compatibility with synthetic hydrocarbon base oil were analyzed by thermogravimetric analysis (TGA) and ultraviolet visible spectroscopy (UV-Vis). The tribological behaviors of DOA-mPEG350 as an ashless friction modifier in synthetic hydrocarbons were studied using a four-ball friction and wear tester. The results show that DOA-mPEG350 has good thermal stability and compatibility with synthetic hydrocarbon base oil, and can effectively shorten the running-in period, and 1.0% (w) addition of DOA-mPEG350 could decrease the ave. coefficient of friction (COF) and wear scar diameter (WSD) of synthetic hydrocarbons such as CTL6 and PAO6 base oils by 20.6%, 20.1% and 28.9%, 34.8%, respectively. The comprehensive friction reduc ing and anti-wear performance of DOA-mPEG350 is better than that of the selected commercial friction modifier and extreme pressure anti-wear additives, which are usually contain sulfur, phosphorus, or metals that can not meet the increasingly stringent environmental requirements, that is, the synthesized DOA-mPEG350 has the potential to replace these additives. Through analyzing the worn friction surface and density functional theory (DFT) calculation, the micro-lubrication mechanism of ether-ester is revealed, namely, it can combine the “line contact” of ether with the “point contact” of ester to form a thick and dense protective film on metal surfaces, thus showing better tribological performance than that only contain ether chain or ester group.
Jun Wang , Xiaomei Xu , Jiaolong Zhou , Yanan Zhao , Xiuli Sun , Yong Tang , Sufang He , Hongmei Yang . Synthesis of New Sulfur-free and Phosphorus-free Ether-ester and Study on Its Properties As Ashless Friction Modifier[J]. Acta Chimica Sinica, 2023 , 81(5) : 461 -468 . DOI: 10.6023/A23030101
| [1] | Ewen, J. P.; Gattinoni, C.; Morgan, N.; Spikes, H.; Dini, D. Langmuir 2016, 32, 4450. |
| [1] | Cyriac, F.; Yi, T. X.; Poornachary, S. K.; Chow, P. S. Tribol. Int. 2022, 165. |
| [2] | Tang, Z. L.; Li, S. H. Curr. Opin. Solid. State Mater. Sci. 2014, 18, 119. |
| [3] | Zhao, X.; Zhang, G.; Wang, L.; Xue, Q. Tribol. Lett. 2017, 65, 64. |
| [4] | De Barros Bouchet, M. I.; Thiebaut, B.; Martin, J. M. RSC Adv. 2015, 5, 93786. |
| [5] | Xu, D.; Wang, C.; Espejo, C.; Wang, J.; Neville, A.; Morina, A. Langmuir 2018, 34, 13523. |
| [6] | De Barros Bouchet, M. I.; Martin, J. M.; Oumahi, C.; Gorbatchev, O.; Afanasiev, P.; Geantet, C.; Iovine, R.; Thiebaut, B.; Heau, C. Tribol. Int. 2018, 119, 600. |
| [7] | Gao, H.; Mcquen, J. S.; Black, E. D. Tribol. T. 2004, 47, 200. |
| [8] | Hu, W. J.; Li, J. S. Acta Chim. Sinica 2022, 80, 310. (in Chinese) |
| [8] | (胡文敬, 李久盛, 化学学报, 2022, 80, 310.) |
| [9] | Zhou, C.; Tan, L.; Hu, W.; Li, J. J. Appl. Polym. Sci. 2020, 138. |
| [10] | Hu, W. J.; Zhang, Z. J.; Zeng, X. Q.; Li, J. S. Ind. Eng. Chem. Res. 2019, 59, 215. |
| [11] | Zheng, D.; Wang, X.; Zhang, M.; Liu, Z.; Ju, C. Tribol. Int. 2019, 130, 324. |
| [12] | Cyriac, F.; Tee, X. Y.; Poornachary, S. K.; Chow, P. S. Friction 2020, 9, 380. |
| [13] | Desanker, M.; He, X. L.; Lu, J.; Johnson, B. A.; Liu, Z.; Delferro, M.; Ren, N.; Lockwood, F. E.; Greco, A.; Erdemir, A.; Marks, T. J.; Wang, Q. J.; Chung, Y. W. Tribol. Lett. 2018, 66, 50. |
| [14] | Spikes, H. Tribol. Lett. 2015, 60, 5. |
| [15] | Jahanmir, S. Wear 1985, 102, 331. |
| [16] | Jahanmir, S.; Beltzer, M. J. Tribol. 1986, 108, 109. |
| [17] | Beltzer, M.; Jahanmir, S. Lubr. Sci. 1988, 1, 3. |
| [18] | Fry, B. M.; Moody, G.; Spikes, H. A.; Wong, J. S. S. Langmuir 2020, 36, 1147. |
| [19] | Nalam, P. C.; Pham, A.; Castillo, R. V.; Espinosa-Marzal, R. M. J. Phys. Chem. C 2019, 123, 13672. |
| [20] | Askwith, T. C.; Cameron, A.; Crouch, R. F. Proc. R Soc. A Math. Phys. Eng. Sci. 1966, 291, 500. |
| [21] | Davidson, J. E.; Hinchley, S. L.; Harris, S. G.; Parkin, A.; Parsons, S.; Tasker, P. A. J. Mol. Graph. Model. 2006, 25, 495. |
| [22] | Simic, R.; Kalin, M. Stroj. Vestn-J. Mech. Eng. 2013, 59, 707. |
| [23] | Luo, Y.; Zhang, S. L.; Xu, R. F.; An, W. J.; Xue, W. G.; Zheng, L. C.; Wang, L. P. Lubricating Oil 2022, 37, 35. (in Chinese) |
| [23] | (罗意, 张少龙, 徐瑞峰, 安文杰, 薛卫国, 郑来昌, 汪利平, 润滑油, 2022, 37, 35.) |
| [24] | Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A. V.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams |
| [25] | Becke, A. D. J. Chem. Phys. 1993, 98, 1372. |
| [26] | Becke, A. D. Phys. Rev. A 1988, 38, 3098. |
| [27] | Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785. |
| [28] | Lu, T.; Chen, F. W. J. Comput. Chem. 2012, 33, 580. |
| [29] | Zhang, J.; Lu, T. Phys. Chem. Chem. Phys. 2021, 23, 20323. |
/
| 〈 |
|
〉 |