

Preparation and Adsorption Properties of ZIF-8@B-CNF Composite Aerogel
Received date: 2023-03-06
Online published: 2023-05-15
Supported by
National Natural Science Foundation of China(22178273); Foundation of Tianjin Key Laboratory of Pulp & Paper (Tianjin University of Science & Technology)(202201)
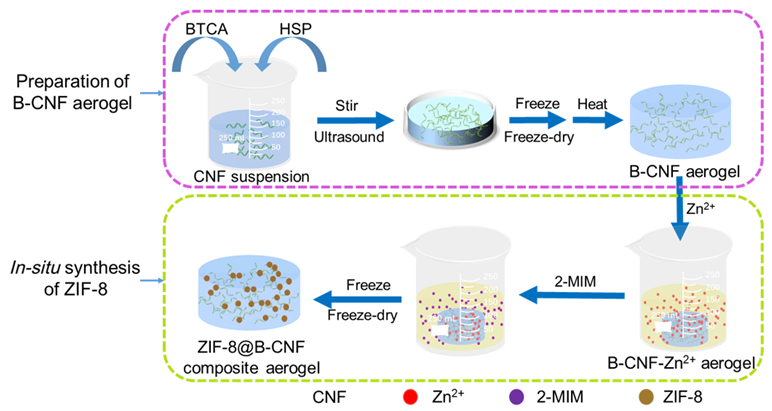
Based on the principle of “fiber first”, cellulose nanofibrils (CNF) and metal-organic framework (MOF) are utilized to prepare composite material. The CNF with high dispersibility was prepared via MgCl2/succinic acid hydrolysis, sodium chlorite oxidation and high-pressure homogenization, then it was chemically cross-linked with 1,2,3,4-butane tetracarboxylic acid (BTCA) and freeze-dried in a mold for 4 h to obtain the aerogel. The aerogel was fully esterified at 170 ℃ for 2~3 min to obtain aerogel B-CNF, then Zn2+ and 2-methylimidazole were synthesized in-situ on its surface, finally, ZIF-8@B-CNF composite aerogel was successfully prepared. After that, the composite aerogel was used for the adsorption of methylene blue (MB), and the effects of adsorption conditions including adsorption time, MB concentration, pH values of the solution were discussed. The ZIF-8@B-CNF composite aerogel was characterized by SEM (scanning electron microscope), XRD (X-ray diffraction), FT-IR (Fourier transform infrared spectroscopy), XPS (X-ray photoelectron spectroscopy), BET (Brunner-Emmet-Teller), etc., and the adsorption behavior and adsorption mechanism of MB were researched. The results showed that the ZIF-8@B-CNF composite aerogel had the advantages of low density and high surface area. When the loading capacity of ZIF-8 came up to 50%, the density of the ZIF-8@B-CNF was as low as less than 0.1 g•cm−3, and its surface area were increased by 14.5 times compared with that of the CNF aerogel. The ZIF-8@B-CNF composite aerogel showed excellent adsorption capacity for the MB, its theoretical maximum adsorption capacity was as high as 352.59 mg•g−1 based on the Langmuir adsorption model, demonstrating that the composite aerogel had excellent adsorption capacity. The adsorption process was in accordance with the quasi-second-order kinetic model, which belonged to the chemisorption. In addition, the adsorption behavior of MB on the composite aerogel mainly depended on the effects of electrostatic interaction, π-π stacking and hydrogen bonding.
Kaiqing Wang , Shuo Yuan , Wangdong Xu , Dan Huo , Qiulin Yang , Qingxi Hou , Dehai Yu . Preparation and Adsorption Properties of ZIF-8@B-CNF Composite Aerogel[J]. Acta Chimica Sinica, 2023 , 81(6) : 604 -612 . DOI: 10.6023/A23020049
| [1] | Li, H.; Eddaoudi, M.; O'keeffe, M.; Yaghi, O. M. Nature 1999, 402, 276. |
| [2] | Zhu, H.; Yang, X.; Cranston, E. D.; Zhu, S. P. Adv. Mater. 2016, 28, 7652. |
| [3] | Lu, L. X.; Zhao, L. Y.; Wei, Y. R.; Wang, H. H. Acta Chim. Sinica 2021, 79, 869. (in Chinese) |
| [3] | (吕露茜, 赵娅俐, 魏嫣莹, 王海辉, 化学学报, 2021, 79, 869.) |
| [4] | Feng, Y.; Li, Y.; Xu, M. Y.; Liu, S. C.; Yao, J. F. RSC Adv. 2016, 6, 109608. |
| [5] | Ahsan, M. A.; Jabbari, V.; El-Gendy, A. A.; Curry, M. L.; Noveron, J. C. Appl. Surf. Sci. 2019, 497, 143608. |
| [6] | Zeng, J. Y.; Wang, X. S.; Zhang, X. Z.; Zhuo, R. X. Acta Chim. Sinica 2019, 77, 1156. (in Chinese) |
| [6] | (曾锦跃, 王小双, 张先正, 卓仁禧, 化学学报, 2019, 77, 1156.) |
| [7] | Li, J. L.; Yuan, S.; Qin, J. S.; Pang, J. D.; Zhang, P.; Zhang, Y. M.; Huang, Y. Y.; Drake, H. F.; Liu, W. S. R.; Zhou, H. C. Angew. Chem. 2020, 132, 9405. |
| [8] | Cheng, P.; Wang, C. H.; Kaneti, Y. V.; Eguchi, M.; Lin, J. J.; Yamauchi, Y.; Na, J. Langmuir 2020, 36, 4231. |
| [9] | Zhang, Z. H.; Zhang, J. L.; Liu, J. M.; Xiong, Z. H.; Chen, X. Water, Air, Soil Pollut. 2016, 227, 471. |
| [10] | Li, Y. F.; Yan, X. L.; Hu, X. Y.; Feng, R.; Zhou, M.; Han, D. Z. J. Porous Mater. 2020, 27, 1109. |
| [11] | Wang, B.; Cote, A. P.; Furukawa, H.; O'Keeffe, M.; Yaghi, O. M. Nature 2008, 453, 207. |
| [12] | Niu, B.; Zhai, Z. Y.; Hao, X. K.; Ren, T. L.; Li, C. J. Acta Chim. Sinica 2022, 80, 946. (in Chinese) |
| [12] | (牛犇, 翟振宇, 郝肖柯, 任婷莉, 李从举, 化学学报, 2022, 80, 946.) |
| [13] | Abdollahi, B.; Najafidoust, A.; Asl, E. A.; Sillanpaa, M. Arabian J. Chem. 2021, 14, 103444. |
| [14] | Isogai, A. J. Wood Sci. 2013, 59, 449. |
| [15] | Du, H. S.; Liu, C.; Zhang, M. M.; Kong, Q. S.; Li, B.; Xian, M. Prog. Chem. 2018, 30, 448. (in Chinese) |
| [15] | (杜海顺, 刘超, 张苗苗, 孔庆山, 李滨, 咸漠, 化学进展, 2018, 30, 448.) |
| [16] | Mondal, S. Carbohydr. Polym. 2017, 163, 301. |
| [17] | Mo, L. T.; Pang, H. W.; Tan, Y.; Zhang, S. F.; Li, J. Z. Chem. Eng. J. 2019, 378, 122157. |
| [18] | Dhali, K.; Ghasemlou, M.; Daver, F.; Cass, P.; Adhikari, B. Sci. Total Environ. 2021, 775, 145871. |
| [19] | Zhu, L. T.; Zong, L.; Wu, X. C.; Li, M. J.; Wang, H. S.; You, J.; Li, C. X. ACS Nano 2018, 12, 4462. |
| [20] | Abdelhamid, H. N.; Mathew, A. P. Chem. Eng. J. 2021, 426, 131733. |
| [21] | Moghaddam, S. S.; Moghaddam, M. R. A.; Arami, M. J. Hazard. Mater. 2010, 175, 651. |
| [22] | Sachdeva, S.; Kumar, A. J. Membr. Sci. 2009, 329, 2. |
| [23] | El-Desoky, H. S.; Ghoneim, M. M.; El-Sheikh, R.; Zidan, N. M. J. Hazard. Mater. 2010, 175, 858. |
| [24] | Li, Z. J.; Zhang, X. W.; Lin, J.; Han, S.; Lei, L. C. Bioresour. Technol. 2010, 101, 4440. |
| [25] | Song, Y. R.; Wang, K. S.; An, G. Y.; Zhao, F. J.; Men, B.; Du, Z. X.; Wang, D. S. Acta Chim. Sinica 2022, 80, 1592. (in Chinese) |
| [25] | (宋亚瑞, 王凯升, 安广宇, 赵法军, 门彬, 杜昭兮, 王东升, 化学学报, 2022, 80, 1592.) |
| [26] | Feng, A. H.; Yu, Y.; Yu, Y.; Song, L. X. Acta Chim. Sinica 2018, 76, 757. (in Chinese) |
| [26] | (冯爱虎, 于洋, 于云, 宋力昕, 化学学报, 2018, 76, 757.) |
| [27] | Peer, F. E.; Bahramifar, N.; Younesi, H. J. Taiwan Inst. Chem. Eng. 2018, 87, 225. |
| [28] | Wang, S. S.; Zhang, L.; Long, C.; Li, A. M. J. Colloid Interface Sci. 2014, 428, 185. |
| [29] | Lu, Y.; Liu, C. Z.; Mei, C. T.; Sun, J. S.; Lee, J.; Wu, Q. L.; Hubbe, M. A.; Li, M. C. Coord. Chem. Rev. 2022, 461, 214496. |
| [30] | Sauperl, O.; Stana-kleinschek, K.; Ribitsch, V. Text. Res. J. 2009, 79, 780. |
| [31] | Ma, X. F.; Liu, C. Z.; Anderson, D. P.; Chang, P. R. Chemosphere 2016, 165, 399. |
| [32] | Liu, Y. P.; Hu, H. Fibers Polym. 2008, 9, 735. |
| [33] | He, M.; Yao, J. F.; Liu, Q.; Wang, K.; Chen, F. Y.; Wang, H. T. Microporous Mesoporous Mater. 2014, 184, 55. |
| [34] | Maiti, S.; Jayaramudu, J.; Das, K.; Reddy, S. M.; Sadiku, R.; Ray, S. S.; Liu, D. G. Carbohydr. Polym. 2013, 98, 562. |
| [35] | Xu, S.; Huo, D.; Wang, K. Q.; Yang, Q. L.; Hou, Q. X.; Zhang, F. S. Carbohydr. Polym. 2021, 266, 118118. |
| [36] | Meng, W. Y.; Wang, S. J.; Lv, H. F.; Wang, Z. X.; Han, X. W.; Zhou, Z. J.; Pu, J. W. Bioresources 2022, 17, 2615. |
| [37] | Zhang, S. H.; Liu, Y.; Li, D.; Wang, Q.; Ran, F. Applied Surface Science 2020, 505, 144533. |
| [38] | Ahmad, R.; Ansari, K. Process Biochem. 2021, 108, 90. |
| [39] | Do, N. H. N.; Truong, B. Y.; Nguyen, P. T. X.; Le, K. A.; Duong, H. M.; Le, P. K. Sep. Purif. Technol. 2022, 283, 120200. |
| [40] | El Haouti, R.; Ouachtak, H.; El Guerdaoui, A.; Amedlous, A.; Amaterz, E.; Haounati, R.; Addi, A. A.; Akbal, F.; El Alem, N.; Taha, M. L. J. Mol. Liq. 2019, 290, 111139. |
| [41] | Chen, W. J.; Ma, H. Z.; Ma, H. Z. Int. J. Biol. Macromol. 2020, 158, 1342. |
| [42] | Zhang, Q. L.; Cheng, Y. L.; Fang, C. Q.; Shi, J. Y.; Chen, J.; Han, H. Z. J. Solid State Chem. 2021, 302, 122361. |
| [43] | Chen, W. J.; Ma, H. Z.; Xing, B. S. Int. J. Biol. Macromol. 2020, 158, 1342. |
| [44] | Abuzerr, S.; Darwish, M.; Mahvi, A. H. Water Sci. Technol. 2018, 2, 534. |
| [45] | Shi, Y. W.; Song, G. B.; Li, A. Q.; Wang, J.; Wang, H. A.; Sun, Y.; Ding, G. H. Colloids Surf., A 2022, 641, 128595. |
| [46] | Zhang, A. J.; Shan, F. J.; Ji, X. Y.; Chen, Y. Q.; Zhao, Y.; Yv, J. New Chem. Mater. 2023, 51, 233. (in Chinese) |
| [46] | (张爱佳, 单凤君, 纪馨越, 陈玥琪, 赵宇, 喻靓, 化工新型材料, 2023, 51, 233.) |
| [47] | Chang, N.; Zhang, H.; Shi, M. S.; Li, J.; Yin, C. J.; Wang, H. T.; Wang, L. Microporous Mesoporous Mater. 2018, 266, 47. |
| [48] | Dai, F. L.; Guo, J. H.; He, Y. F.; Song, P. F.; Wang, R. M. Clay Minerals 2021, 56, 99. |
| [49] | Zhang, Q. L.; Cheng, Y. L.; Fang, C. Q.; Shi, J. Y.; Chen, J.; Han, H. Z. J. Solid State Chem. 2021, 299, 122190. |
| [50] | Zhang, H.; Zhao, M.; Yang, Y.; Lin, Y. S. Microporous Mesoporous Mater. 2019, 288, 109568. |
| [51] | Liang, Y. X.; Li, H. B.; Li, X. T.; Zhang, Q. Y.; Fei, J. Y.; Li, S. M.; Chen, S. Ecotoxicol. Environ. Saf. 2022, 113450. |
| [52] | Karami, A.; Shomal, R.; Sabouni, R.; Al-Sayah, M. H.; Aidan, A. Energ. 2022, 15, 4642. |
| [53] | Nazir, M. A.; Najam, T.; Zarin, K.; Shahzad, K.; Javed, M. S.; Jamshaid, M.; Bashir, M. A.; Shah, S. S. A.; Rehman, A. U. Int. J. Environ. Anal. Chem. 2021, 3, 1931855. |
| [54] | Foo, K. Y.; Hameed, B. H. Chem. Eng. J. 2010, 156, 2. |
| [55] | Iftekhar, S.; Ramasamy, D. L.; Srivastava, V.; Asif, M. B.; Sillanpaa, M. Chemosphere 2018, 204, 413. |
| [56] | Febrianto, J.; Kosasih, A. N.; Sunarso, J.; Ju, Y. H.; Indraswati, N.; Ismadji, S. J. Hazard. Mater. 2009, 162, 616. |
| [57] | Qiu, H.; Lv, L.; Pan, B. C.; Zhang, Q. J.; Zhang, W. M.; Zhang, Q. X. J. Zhejiang Univ.-Sci. A 2009, 10, 716. |
/
| 〈 |
|
〉 |