

Design, Synthesis and Electroluminescence Performance of Flexible Fluorenyl Block Delayed Fluorescence Dimers
Received date: 2023-02-26
Online published: 2023-05-23
Supported by
The National Natural Science Foundation of China(21805106); Lianyungang Project for Transformation of Scientific and Technological Achievements(CA202103); Lianyungang 521 Funding Project(LYG06521202161); Six Talent Peaks Project in Jiangsu Province(JNHB 114)
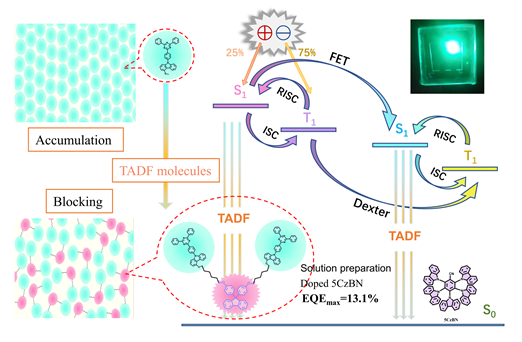
Organic light-emitting diodes (OLEDs) have a series of advantages such as high resolution, wide viewing angle, lightweight, and flexible solution processing that can realize a large area of self-illumination display. As the third generation of luminescence materials, thermally-activated delayed fluorescence (TADF) material can harvest 100% of triplet excitons through reverse intersystem crossing (RISC). However, the triplet excitons exhibit severe aggregation-caused quenching (ACQ) behavior in the tightly stacked state, which undoubtedly reduces the device’s performance. In this work, we synthesized the block dimer TADF material 2TC-Fu, among the molecular structure, diphenylfluorene serves as the block framework and triazinocarbazole (TRZ-CZ) serves as the TADF emissive unit. First, carbazole is connected to the end of 1,6-dibromohexane, and then the symmetric structure is synthesized from nucleophilic substituted bisphenol fluorene, after, monofluorotriazine is attached to both ends of the symmetric structure by a nucleophilic substitution reaction to generate the target product 2TC-Fu finally. Because the addition of diphenyl fluorene obviously shows a spatial torsion angle, the irregular distribution of the emitting unit is realized by bridging the flexible alkyl chain in the middle of TADF, thus effectively avoiding the ACQ of the TADF unit and improving its solubility in the solvent. Thermodynamic properties show that 2TC-Fu has a high glass transition temperature (142 ℃) and a thermal decomposition temperature of 442 ℃, proving that it can be sufficient to withstand the heat treatment procedures during device preparation. In addition, the device 2TC-Fu based exhibits a high CEmax and EQEmax of 8.3 cd/A and 4.4% respectively, which exceeds 3 times the efficiency of non-block TADF unit-based, demonstrating the feasibility of the flexible fluorenyl block strategy. Moreover, the devices 2TC-Fu doped with TADF material 5CzBN achieved 13.1% of EQEmax and 3489 cd/m2 of brightness. In summary, the design of a flexible fluorenyl block strategy to avoid large-scale aggregation of luminescent nuclei significantly improves the device efficiency, which provides a new way for the development of TADF molecules. Although the aggregation-induced fluorescence quench-ing was effectively inhibited by increasing the molecular spacing between luminescent units and increasing the solubility of molecules by using large fluorenyl groups and alkoxy chains, we will not stop researching the blocking strategy. In the future, we will continue to explore the factors that affect the performance of TADF devices in all aspects, such as the type of block groups and the steric hindrance of substituents.
Fengjie Ge , Kaizhi Zhang , Qingpeng Cao , Hui Xu , Tao Zhou , Wenhao Zhang , Xinxin Ban , Xiaobo Zhang , Na Li , Peng Zhu . Design, Synthesis and Electroluminescence Performance of Flexible Fluorenyl Block Delayed Fluorescence Dimers[J]. Acta Chimica Sinica, 2023 , 81(9) : 1157 -1166 . DOI: 10.6023/A23020051
| [1] | Wong, M. Y.; Zysman-Colman, E. Adv. Mater. 2017, 29, 1605444. |
| [2] | Krucaite, G.; Grigalevicius, S. Synth Met 2019, 247, 90. |
| [3] | Manikandan, M.; Nirmal, D.; Ajayan, J.; Mohankumar, P.; Prajoon, P.; Arivazhagan, L. Superlattices Microstruct 2019, 136, 106294. |
| [4] | Ràfols-Ribé, J.; Will, P.-A.; H?nisch, C.; Gonzalez-Silveira, M.; Lenk, S.; Rodríguez-Viejo, J.; Reineke, S. Sci. Adv. 2018, 4, eaar8332. |
| [5] | Zhang, L.; Li, M.; Gao, Q. Y.; Chen, C. F. Chin J. Org. Chem 2020, 40, 516. (in Chinese) |
| [5] | 张亮, 李猛, 高庆宇, 陈传峰, 有机化学, 2020, 40, 516). |
| [6] | Zhang, S. Q.; Li, M. Q.; Zhou, Z. J.; Qu, Z. X. Acta Chim Sinica 2023, 81, 124. (in Chinese) |
| [6] | 张少秦, 李美清, 周中军, 曲泽星, 化学学报, 2023, 81, 124). |
| [7] | Tan, H.-J.; Yang, G.-X.; Deng, Y.-L.; Cao, C.; Tan, J.-H.; Zhu, Z.-L.; Chen, W.-C.; Xiong, Y.; Jian, J.-X.; Lee, C.-S.; Tong, Q.-X. Adv. Mater. 2022, 34, 2200537. |
| [8] | Zhang, Q.; Li, B.; Huang, S.; Nomura, H.; Tanaka, H.; Adachi, C. Nat. Photonics 2014, 8, 326. |
| [9] | Ogorodnikov, I. N.; Kiseleva, M. S.; Yakovlev, V. Y. Opt. Mater. 2012, 34, 2030. |
| [10] | Vázquez, R. J.; Yun, J. H.; Muthike, A. K.; Howell, M.; Kim, H.; Madu, I. K.; Kim, T.; Zimmerman, P.; Lee, J. Y.; Iii, T. G. J. Am. Chem. Soc. 2020, 142, 8074. |
| [11] | Samanta, P. K.; Kim, D.; Coropceanu, V.; Brédas, J.-L. J. Am. Chem. Soc. 2017, 139, 4042. |
| [12] | Zhang, Q.; Li, J.; Shizu, K.; Huang, S.; Hirata, S.; Miyazaki, H.; Adachi, C. J. Am. Chem. Soc. 2012, 134, 14706. |
| [13] | Nakanotani, H.; Furukawa, T.; Morimoto, K.; Adachi, C. Sci. Adv. 2016, 2, e1501470. |
| [14] | Cravcenco, A.; Hertzog, M.; Ye, C.; Iqbal, M. N.; Mueller, U.; Eriksson, L.; B?rjesson, K. Sci. Adv. 2019, 5, eaaw5978. |
| [15] | Wang, J.; Li, N.; Chen, Q.; Xiang, Y.; Zeng, X.; Gong, S.; Zou, Y.; Liu, Y. Chem. Eng. J. 2022, 450, 137805. |
| [16] | Ikeda, N.; Oda, S.; Matsumoto, R.; Yoshioka, M.; Fukushima, D.; Yoshiura, K.; Yasuda, N.; Hatakeyama, T. Adv. Mater. 2020, 32, 2004072. |
| [17] | Rajamalli, P.; Senthilkumar, N.; Huang, P. Y.; Ren-Wu, C. C.; Lin, H. W.; Cheng, C. H. J. Am. Chem. Soc. 2017, 139, 10948. |
| [18] | Aizawa, N.; Matsumoto, A.; Yasuda, T. Sci. Adv. 2021, 7, eabe5769. |
| [19] | Phan Huu, D. K. A.; Saseendran, S.; Dhali, R.; Franca, L. G.; Stavrou, K.; Monkman, A.; Painelli, A. J. Am. Chem. Soc. 2022, 144, 15211. |
| [20] | Bezvikonnyi, O.; Gudeika, D.; Volyniuk, D.; Bucinskas, A.; Grazulevicius, J. V. Mater. Sci. Eng. B 2021, 273, 115441. |
| [21] | Fu, Y.; Liu, H.; Yang, D.; Ma, D.; Zhao, Z.; Tang, B. Z. Sci. Adv. 2021, 7, eabj2504. |
| [22] | Kim, E.; Park, J.; Jun, M.; Shin, H.; Baek, J.; Kim, T.; Kim, S.; Lee, J.; Ahn, H.; Sun, J.; Ko, S.-B.; Hwang, S.-H.; Lee, J. Y.; Chu, C.; Kim, S. Sci. Adv. 2022, 8, eabq1641. |
| [23] | Kawasumi, K.; Wu, T.; Zhu, T.; Chae, H. S.; Van Voorhis, T.; Baldo, M. A.; Swager, T. M. J. Am. Chem. Soc. 2015, 137, 11908. |
| [24] | Li, H.; Wang, Y.; Yu, L.; Liu, C.; Zhou, C.; Sun, S.; Li, M.; Tao, Y.; Xie, G.; Xu, H.; Huang, W.; Chen, R. Chem. Eng. J. 2021, 425, 131487. |
| [25] | Manivannan, R.; Park, S. H.; Oh, H.; Son, Y.-A. Dyes Pigm. 2022, 205, 110480. |
| [26] | Lv, X.; Wang, Y.; Li, N.; Cao, X.; Xie, G.; Huang, H.; Zhong, C.; Wang, L.; Yang, C. Chem. Eng. J. 2020, 402, 126173. |
| [27] | Li, N.; Chai, D.; Chen, Z.; Zhou, C.; Ni, F.; Huang, Z.; Cao, X.; Xie, G.; Li, K.; Yang, C. Chem. Eng. J. 2020, 396, 125276. |
| [28] | Liang, Z. P.; Tang, R.; Qiu, Y. C.; Wang, Y.; Lu, H. B.; Wu, Z. G. Acta Chim. Sinica 2021, 79, 1401. (in Chinese) |
| [28] | 梁志鹏, 唐瑞, 邱雨晨, 王阳, 陆洪彬, 吴正光, 化学学报, 2021, 79, 1401). |
| [29] | Tao, Y.; Yuan, K.; Chen, T.; Xu, P.; Li, H.; Chen, R.; Zheng, C.; Zhang, L.; Huang, W. Adv. Mater. 2014, 26, 7931. |
| [30] | Lee, S. Y.; Adachi, C.; Yasuda, T. Adv. Mater. 2016, 28, 4626. |
| [31] | Ha, T. H.; Bin, J.-K.; Lee, C. W. Org. Electron. 2022, 102, 106450. |
| [32] | Armakovi?, S. J.; Mary, Y. S.; Mary, Y. S.; Pelemi?, S.; Armakovi?, S. Comput. Theor. Chem. 2021, 1197, 113160. |
| [33] | Skuodis, E.; Bezvikonnyi, O.; Tomkeviciene, A.; Volyniuk, D.; Mimaite, V.; Lazauskas, A.; Bucinskas, A.; Keruckiene, R.; Sini, G.; Grazulevicius, J. V. Org. Electron. 2018, 63, 29. |
| [34] | Moon, C.-K.; Suzuki, K.; Shizu, K.; Adachi, C.; Kaji, H.; Kim, J.-J. Adv. Mater. 2017, 29, 1606448. |
| [35] | Yang, H.-Y.; Zhang, M.; Zhao, J.-W.; Pu, C.-P.; Lin, H.; Tao, S.-L.; Zheng, C.-J.; Zhang, X.-H. Chin. J. Chem. 2022, 40, 911. |
| [36] | Wang, T. T.; Hua, X. C.; Yu, Y. J.; Yuan, Y.; Fung, M. Q.; Jiang, Z. Q. Chin J. Org. Chem 2019, 39, 1436. (in Chinese) |
| [36] | 王彤彤, 华晓晨, 郁友军, 袁熠, 冯敏强, 蒋佐权, 有机化学, 2019, 39, 1436). |
| [37] | Hong, G.; Gan, X.; Leonhardt, C.; Zhang, Z.; Seibert, J.; Busch, J. M.; Br?se, S. Adv. Mater. 2021, 33, 2005630. |
| [38] | Liu, Y.; Li, C.; Ren, Z.; Yan, S.; Bryce, M. R. Nat. Rev. Mater. 2018, 3, 18020. |
| [39] | Cao, X.; Chen, Z.; Gong, S.; Pan, K.; Zhou, C.; Huang, T.; Chai, D.; Zhan, Q.; Li, N.; Zou, Y.; Liu, H.; Yang, C. Chem. Eng. J. 2020, 399, 125648. |
| [40] | Al-Otaibi, J. S.; Mary, Y. S.; Mary, Y. S.; Soman, S.; Acharjee, N.; Narayana, B. Chem. Phys. Lett. 2022, 793, 139469. |
| [41] | Yersin, H.; Czerwieniec, R.; Monkowius, U.; Ramazanov, R.; Valiev, R.; Shafikov, M. Z.; Kwok, W.-M.; Ma, C. Coord. Chem. Rev. 2023, 478, 214975. |
| [42] | Sun, D.; Duda, E.; Fan, X.; Saxena, R.; Zhang, M.; Bagnich, S.; Zhang, X.; Kohler, A.; Zysman-Colman, E. Adv. Mater. 2022, 34, e2110344. |
| [43] | Wei, J.; Liu, D.; Sun, K.; Tian, W.; Jiang, W.; Sun, Y. Dyes Pigm. 2020, 182, 108624. |
| [44] | Liu, B.; Li, J.; Liu, D.; Mei, Y.; Lan, Y.; Song, K.; Li, Y.; Wang, J. Dyes Pigm. 2022, 203, 110329. |
| [45] | Shin, K.; Lee, E.; Lee, T.; Lee, Y. H.; Kim, D. H.; Kim, C.; Jung, J.; Jung, B. J.; Lee, M. H. Dyes Pigm. 2023, 209, 110937. |
| [46] | Chen, Y.; Li, N.; Huang, Z.; Xie, G.; Yang, C. Chem. Eng. J. 2022, 430, 133078. |
| [47] | Wang, T.-T.; Xie, G.; Li, H.-C.; Yang, S.-Y.; Li, H.; Xiao, Y.-L.; Zhong, C.; Sarvendra, K.; Khan, A.; Jiang, Z.-Q.; Liao, L.-S. CCS Chemistry 2021, 3, 1757. |
| [48] | Zhang, K.; Zhou, T.; Cao, Q.; Ge, F.; Xu, H.; Chu, J.; Wang, J.; Pei, M.; Ban, X.; Zhang, T. Org. Electron. 2023, 112, 106687. |
| [49] | Pathak, S. K.; Liu, H.; Zhou, C.; Xie, G.; Yang, C. J. Mater. Chem. C 2021, 9, 7363. |
| [50] | Zhao, G.; Liu, D.; Wang, P.; Huang, X.; Chen, H.; Zhang, Y.; Zhang, D.; Jiang, W.; Sun, Y.; Duan, L. Angew. Chem. Int. Ed. 2022, 61, e202212861. |
| [51] | Liang, J.-J.; Li, Y.; Yuan, Y.; Li, S.-H.; Zhu, X.-D.; Barlow, S.; Fung, M.-K.; Jiang, Z.-Q.; Marder, S. R.; Liao, L.-S. Mater. Chem. Front. 2018, 2, 917. |
| [52] | Niu, R.; Li, J.; Liu, D.; Dong, R.; Wei, W.; Tian, H.; Shi, C. Dyes Pigm. 2021, 194, 109581. |
| [53] | Li, Y.; Liang, J. J.; Li, H. C.; Cui, L. S.; Fung, M. K.; Barlow, S.; Marder, S. R.; Adachi, C.; Jiang, Z. Q.; Liao, L. S. J. Mater. Chem. C 2018, 6, 5536. |
/
| 〈 |
|
〉 |