

Cobalt-Substituted Polyoxometalates as Soluble Mediators to Boost the Lithium-Sulfur Battery Performance
Received date: 2023-03-18
Online published: 2023-05-26
Supported by
National Natural Science Foundation of China(21832003); Natural Science Foundation of Jiangsu Province, Major Project(BK20212005)
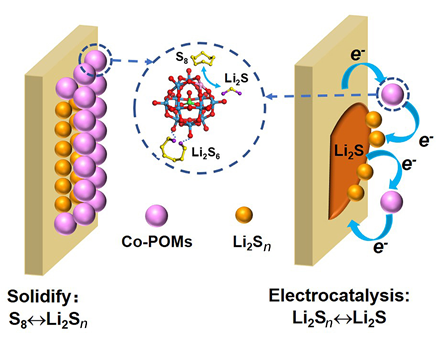
Lithium-sulfur battery (LSB) is a kind of promising next-generation high-energy secondary battery due to its high theoretical energy density, low cost and environmental friendliness. Many studies have been devoted to solve the challenging problems faced by LSB such as the shuttle effect of soluble lithium polysulfides (Li2Sn, 8≥n>2) and the polarization effect of sluggish S8↔Li2S conversion. Currently, the main strategies to cope with these challenges include cathode structure design, separator modification and electrolyte regulation, etc., all of which can suppress the shuttle and polarization effects to some extend and thereby optimize the performance of LSB. Adding soluble mediators into electrolytes is a convenient approach, and the key is to develop advanced soluble mediators with synergic functions of inhibiting the polysulfides shuttle and promoting the sulfur/sulfides conversion kinetics. Polyoxometalates (POMs) have unique redox properties and are widely used in the fields of photocatalysis, fuel cells, supercapacitors and rechargeable batteries. POMs have excellent electrochemical stability, and their redox reaction potentials well match the potentials of sulfur/sulfides conversion reactions. Therefore, the utilization of POMs in LSB has great prospect, but is still in its infancy. Herein, cobalt-substituted polyoxometalates (Co-POMs) are used as the soluble mediator of LSB for the first time, which can solidify the soluble polysulfides by chemical adsorption and promote the reversible conversion of S8↔Li2S by electrocatalysis simultaneously, which effectively suppress the shuttle and polarization effects, leading to the improved performance of LSB. The LSB with Co-POMs remains the high specific capacity of 565 mAh•g-1 after 400 cycles at 2 A•g-1 and the coulombic efficiency close to 100%. At a high rate of 5 A•g-1, it still shows a discharge capacity of 518 mAh•g-1, obviously better than the LSB with tetrabutylammonium-POMs or without mediators. This study provides a new strategy to improve the LSB performance by developing advanced quantum dot-type soluble mediators with dual-functional adsorption and electrocatalysis.
Ziqi Li , Liwei Liu , Chenghui Mao , Changkai Zhou , Minqi Xia , Zhen Shen , Yue Guo , Qiang Wu , Xizhang Wang , Lijun Yang , Zheng Hu . Cobalt-Substituted Polyoxometalates as Soluble Mediators to Boost the Lithium-Sulfur Battery Performance[J]. Acta Chimica Sinica, 2023 , 81(6) : 620 -626 . DOI: 10.6023/A23030083
| [1] | Seh, Z. W.; Sun, Y.; Zhang, Q.; Cui, Y. Chem. Soc. Rev. 2016, 45, 5605. |
| [2] | Yang, X.; Li, X.; Adair, K.; Zhang, H.; Sun, X. Electrochem. Energy Rev. 2018, 1, 239. |
| [3] | Wang, X.; Li, Y.; Du, L.; Gao, F.; Wu, Q.; Yang, L.; Chen, Q.; Wang, X.; Hu, Z. Acta Chim. Sinica 2018, 76, 627. (in Chinese) |
| [3] | (王啸, 李有彬, 杜玲玉, 高福杰, 吴强, 杨立军, 陈强, 王喜章, 胡征, 化学学报, 2018, 76, 627.) |
| [4] | Barghamadi, M.; Best, A. S.; Bhatt, A. I.; Hollenkamp, A. F.; Musameh, M.; Rees, R. J.; Rüther, T. Energy Environ. Sci. 2014, 7, 3902. |
| [5] | Liu, L.; Guo, Y.; Qi, Z.; Zeng, Y.; Cheng, X.; Yang, L.; Wu, Q.; Wang, X.; Hu, Z. Small Struct. 2022, 3, 2200050. |
| [6] | He, J.; Jiao, L.; Cheng, X.; Chen, G.; Wu, Q.; Wang, X.; Yang, L.; Hu, Z. Acta Chim. Sinica 2022, 80, 896. (in Chinese) |
| [6] | (何家伟, 焦柳, 程雪怡, 陈光海, 吴强, 王喜章, 杨立军, 胡征, 化学学报, 2022, 80, 896.) |
| [7] | Lei, T.; Chen, W.; Lv, W.; Huang, J.; Zhu, J.; Chu, J.; Yan, C.; Wu, C.; Yan, Y.; He, W.; Xiong, J.; Li, Y.; Yan, C.; Goodenough, J. B.; Duan, X. Joule 2018, 2, 2091. |
| [8] | Deng, C.; Wang, Z.; Wang, S.; Yu, J.; Martin, D. J.; Nanjundan, A. K.; Yamauchi, Y. ACS Appl. Mater. Interfaces 2019, 11, 541. |
| [9] | Gu, S.; Wen, Z.; Qian, R.; Jin, J.; Wang, Q.; Wu, M.; Zhuo, S. ACS Appl. Mater. Interfaces 2016, 8, 34379. |
| [10] | Hu, C.; Chen, H.; Shen, Y.; Lu, D.; Zhao, Y.; Lu, A. H.; Wu, X.; Lu, W.; Chen, L. Nat. Commun. 2017, 8, 479. |
| [11] | Yang, T.; Qian, T.; Liu, J.; Xu, N.; Li, Y.; Grundish, N.; Yan, C.; Goodenough, J. B. ACS Nano 2019, 13, 9067. |
| [12] | Liu, G.; Sun, Q.; Li, Q.; Zhang, J.; Ming, J. Energy Fuels 2021, 35, 10405. |
| [13] | Aurbach, B.; Pollak, E.; Elazari, R.; Salitra, G.; Kelley, C. S.; Affinito, J. J. Electrochem. Soc. 2009, 156, A694. |
| [14] | Tsao, Y.; Lee, M.; Miller, E. C.; Gao, G.; Park, J.; Chen, S.; Katsumata, T.; Tran, H.; Wang, L. W.; Toney, M. F.; Cui, Y.; Bao, Z. Joule 2019, 3, 872. |
| [15] | Zhao, M.; Peng, H. J.; Wei, J. Y.; Huang, J. Q.; Li, B. Q.; Yuan, H.; Zhang, Q. Small Methods 2020, 4, 1900344. |
| [16] | Xu, Z. L.; Lin, S.; Onofrio, N.; Zhou, L.; Shi, F.; Lu, W.; Kang, K.; Zhang, Q.; Lau, S. P. Nat. Commun. 2018, 9, 4164. |
| [17] | Li, F.; Zhang, M.; Chen, W.; Cai, X.; Rao, H.; Chang, J.; Fang, Y.; Zhong, X.; Yang, Y.; Yang, Z.; Yu, X. ACS Appl. Mater. Interfaces 2021, 13, 30746. |
| [18] | Fu, Y.; Wu, Z.; Yuan, Y.; Chen, P.; Yu, L.; Yuan, L.; Han, Q.; Lan, Y.; Bai, W.; Kan, E.; Huang, C.; Ouyang, X.; Wang, X.; Zhu, J.; Lu, J. Nat. Commun. 2020, 11, 845. |
| [19] | Liu, W.; Mu, W.; Liu, M.; Zhang, X.; Cai, H.; Deng, Y. Nat. Commun. 2014, 5, 3208. |
| [20] | Homewood, T.; Frith, J. T.; Vivek, J. P.; Casan-Pastor, N.; Tonti, D.; Owen, J. R.; Garcia-Araez, N. Chem. Commun. 2018, 54, 9599. |
| [21] | Yu, B.; Zhang, S.; Wang, X. Angew. Chem., Int. Ed. 2021, 60, 17404. |
| [22] | Hwang, S. K.; Patil, S. J.; Chodankar, N. R.; Huh, Y. S.; Han, Y. K. Chem. Eng. J. 2022, 427, 131854. |
| [23] | Liu, Q.; Wang, X. InfoMat 2021, 3, 854. |
| [24] | Liu, Q.; Yu, H.; Zhang, Q.; Wang, D.; Wang, X. Adv. Funct. Mater. 2021, 31, 2103561. |
| [25] | Ye, J. C.; Chen, J. J.; Yuan, R. M.; Deng, D. R.; Zheng, M. S.; Cronin, L.; Dong, Q. F. J. Am. Chem. Soc. 2018, 140, 3134. |
| [26] | Ni, L.; Yang, G.; Liu, Y.; Wu, Z.; Ma, Z.; Shen, C.; Lv, Z.; Wang, Q.; Gong, X.; Xie, J.; Diao, G.; Wei, Y. ACS Nano 2021, 15, 12222. |
| [27] | Choi, W.; Im, D.; Park, M. S.; Ryu, Y. G.; Hwang, S. S.; Kim, Y. S.; Kim, H.; Doo, S. G.; Chang, H. Electrochemistry 2016, 84, 882. |
| [28] | Zhou, G.; Liu, Y.; Xian, M.; Wang, J.; Zhuang, H.; Cheng, T.; Li, W.; Bi, Y.; Zhen, K. J. Fuel Chem. Technol. 2001, 29, 205. (in Chinese) |
| [28] | (周广栋, 刘延, 咸漠, 王君霞, 庄红, 程铁欣, 李文兴, 毕颖丽, 甄开吉, 燃料化学学报, 2001, 29, 205.) |
| [29] | Xue, X.; Zhao, W.; Ma, B.; Ding, Y. Catal. Commun. 2012, 29, 73. |
| [30] | Ensafi, A. A.; Heydari-Soureshjani, E.; Rezaei, B. Electrochim. Acta 2018, 289, 13. |
| [31] | Ma, J.; Ye, X.; Wu, Y. Chin. J. Catal. 1991, 12, 443. (in Chinese) |
| [31] | (马建伟, 叶兴凯, 吴越, 催化学报, 1991, 12, 443.) |
| [32] | Liu, Q.; He, S.; Yu, B.; Cheng, X.; Shi, W.; Wang, X. Adv. Mater. 2022, 34, 2206178. |
| [33] | Gamelas, J. A. F.; Soares, M. R.; Ferreira, A.; Cavaleiro, A. M. V. Inorg. Chim. Acta 2003, 342, 16. |
| [34] | Chen, C. H.; Lin, S. H.; Wu, Y. J.; Su, J. T.; Cheng, C. C.; Cheng, P. Y.; Ting, Y. C.; Lu, S. Y. Chem. Eng. J. 2022, 431, 133924. |
| [35] | Wang, B.; Wang, X.; Cheng, X.; Zhang, J.; Yan, M.; Li, G.; Yang, L.; Wu, Q.; Wang, X.; Hu, Z. CCS Chem. 2020, 2, 1350. |
| [36] | Wang, W. P.; Zhang, J.; Yin, Y. X.; Duan, H.; Chou, J.; Li, S. Y.; Yan, M.; Xin, S.; Guo, Y. G. Adv. Mater. 2020, 32, 2000302. |
| [37] | Chung, S. H.; Luo, L.; Manthiram, A. ACS Energy Lett. 2018, 3, 568. |
| [38] | Huang, Y.; Chen, S.; Wu, Z.; Wang, J.; Deng, Q.; Zeng, Z.; Deng, S. Electrochim. Acta 2020, 343, 136148. |
| [39] | Ensafi, A. A.; Heydari-Soureshjani, E.; Rezaei, B. Int. J. Hydrogen Energy 2017, 42, 5026. |
| [40] | Lee, J. S.; Lee, C.; Lee, J. Y.; Ryu, J.; Ryu, W. H. ACS Catal. 2018, 8, 7213. |
| [41] | Liu, Q.; Wang, X. Angew. Chem., Int. Ed. 2023, 62, e202217764. |
| [42] | Fan, F. Y.; Carter, W. C.; Chiang, Y. M. Adv. Mater. 2015, 27, 5203. |
| [43] | Yuan, H.; Peng, H. J.; Li, B. Q.; Xie, J.; Kong, L.; Zhao, M.; Chen, X.; Huang, J. Q.; Zhang, Q. Adv. Energy Mater. 2019, 9, 1802768. |
/
| 〈 |
|
〉 |