

Catalytic Synthesis of Polyolefin Elastomer Using Unsymmetrical α-Diimine Nickel Catalyst★
Received date: 2023-04-25
Online published: 2023-06-02
Supported by
National Natural Science Foundation of China(52025031); National Natural Science Foundation of China(U19B6001); National Natural Science Foundation of China(U1904212)
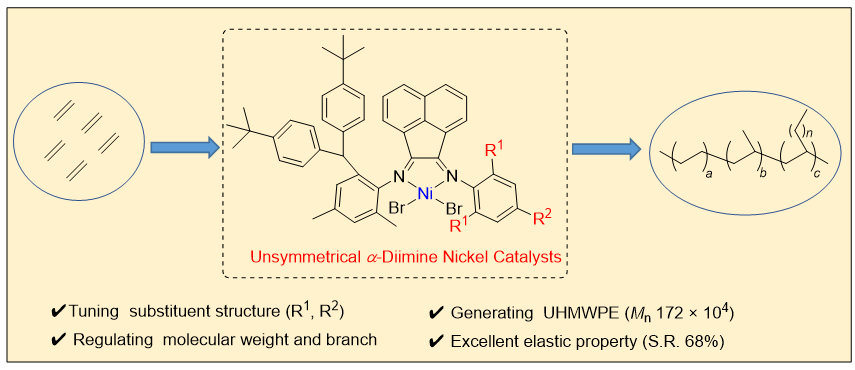
Since the discovery of Ziegler-Natta catalyst, great breakthrough has been made in the design of catalyst in olefin polymerization field, but the development of catalyst is still the fresh source in this field. It will always be a frontier scientific problem in this field to influence the olefin polymerization process through the structural design of catalysts. Due to the low cost of nickel and its high abundance, nickel-based catalysts have shown great prospects in the application of olefin polymerization. In this work, three kinds of unsymmetrical α-diimine nickel catalysts with different steric effects were designed, and their catalytic performances in ethylene polymerization were explored. It is found that the variety of catalysts and the change of polymerization conditions have important effects on the catalytic activity of ethylene polymerization, the molecular weight, branching density, thermodynamic parameters and mechanical properties of the prepared polyethylene. Among them, the polyolefin prepared by Ni3 catalyst with large steric hindrance is ultra-high molecular weight polyethylene, and the above materials maintain excellent mechanical properties and elastic properties at the same time. Specifically, tert-butyl substituted ketoimine intermediate B was condensed with three different anilines to synthesize ligands L1~L3 with different steric effects. The catalysts Ni1~Ni3 were successfully synthesized by efficient coordination reaction between the obtained ligands and DMENiBr2, and the yields were over 90%. The single crystal structures of catalysts Ni1 and Ni3 were also prepared, and the steric hindrance around the nickel center was quantified utilizing the steric maps [Vbur(Ni1)=46.1%, Vbur(Ni3)=48.7%]. Under 0.8 MPa pressure of ethylene and AlEt2Cl as cocatalyst, the ethylene polymerization performance was explored using three nickel catalysts at different temperatures. Among them, Ni2 exhibited the best catalytic activity in ethylene polymerization at 50 ℃ (up to 1.7×107 g•mol-1•h-1), generating polyethylene with higher branching density. The polyethylene prepared by Ni3 with large steric hindrance is ultra-high molecular weight polyethylene with a molecular weight of 172×104 g•mol-1. Moreover, polyethylene materials prepared by Ni3 also showed excellent mechanical properties (breaking stress, 6~8 MPa; elongation at break, ≈600%) and elastic properties (strain recovery value up to 68%).
Zihao Wang , Min Chen , Changle Chen . Catalytic Synthesis of Polyolefin Elastomer Using Unsymmetrical α-Diimine Nickel Catalyst★[J]. Acta Chimica Sinica, 2023 , 81(6) : 559 -564 . DOI: 10.6023/A23040162
| [1] | Sturzel, M.; Mihan, S.; Mulhaupt, R. Chem. Rev. 2016, 116, 1398. |
| [2] | Cui, D. M. Acta Polym. Sinica 2020, 51, 12. (in Chinese) |
| [2] | (崔冬梅, 高分子学报, 2020, 51, 12.) |
| [3] | Jian, Z. B. Acta Polym. Sinica 2018, (11), 1359. (in Chinese) |
| [3] | (简忠保, 高分子学报, 2018, (11), 1359.) |
| [4] | Chen, M.; Chen, C. L. Acta Polym. Sinica 2018, (11), 1372. (in Chinese) |
| [4] | (陈敏, 陈昶乐, 高分子学报, 2018, (11), 1372.) |
| [5] | Tan, C.; Chen, C. L. Angew. Chem., Int. Ed. 2019, 58, 7192. |
| [6] | Li, Y.; Wang, X. Y.; Tang, Y. Acta Chim. Sinica 2021, 79, 1320. (in Chinese) |
| [6] | (李勇, 王晓艳, 唐勇, 化学学报, 2021, 79, 1320.) |
| [7] | Peng, W.; Qi, P. Y.; Dong, K. X.; He, A. H. Acta Chim. Sinica 2020, 78, 1418. (in Chinese) |
| [7] | (彭伟, 戚佩瑶, 董凯旋, 贺爱华, 化学学报, 2020, 78, 1418.) |
| [8] | Mu, H.; Pan, L.; Li, Y. Chem. Rev. 2015, 115, 12091. |
| [9] | Mu, H. L.; Zhou, G. L.; Hu, X. Q.; Jian, Z. B. Coord. Chem. Rev. 2021, 435, 213802. |
| [10] | Chen, C. Nat. Rev. Chem. 2018, 2, 6. |
| [11] | Fu, L. R.; Wang, Y. B.; Jiang, H.; Hao, X. Q.; Song, M. P. Chin. J. Org. Chem. 2022, 42, 3530. (in Chinese) |
| [11] | (付联荣, 王艳冰, 姜辉, 郝新奇, 宋毛平, 有机化学, 2022, 42, 3530.) |
| [12] | Wang, Y.; Yan, J. L. Acta Chim. Sinica 2023, 81, 275. (in Chinese) |
| [12] | (汪阳, 阎敬灵, 化学学报, 2023, 81, 275.) |
| [13] | Zhang, Y. X.; Zhang, Y. X.; Hu, X. Q.; Wang, C. Q.; Jian, Z. B. ACS Catal. 2022, 12, 14304. |
| [14] | Wang, H. B.; Yang, Y.; Nishiura, M.; Higaki, Y.; Takahara, A.; Hou, Z. M. J. Am. Chem. Soc. 2019, 141, 3249. |
| [15] | Wu, Y.; Nan, T. H.; Ji, X. L.; Liu, B.; Cui, D. M. Angew. Chem., Int. Ed. 2022, 61, e202205894 |
| [16] | Jiang, Y.; Zhang, Z.; Li, S. H.; Cui, D. M. Angew. Chem., Int. Ed. 2022, 61, e202112966. |
| [17] | Ji, G.; Chen, Z.; Wang, X. Y.; Ning, X. S.; Xu, C. J.; Zhang, X. M.; Tao, W. J.; Li, J. F.; Gao, Y. S.; Shen, Q.; Sun, X. L.; Wang, H. Y.; Zhao, J. B.; Zhang, B.; Guo, Y. L.; Zhao, Y. N.; Sun, J. J.; Luo, Y.; Tang, Y. Nat. Commun. 2021, 12, 1. |
| [18] | Tran, T. V.; Do, L. H. Eur. Polym. J. 2021, 142, 110100. |
| [19] | Johnson, L. K.; Killian, C. M.; Brookhart, M. J. Am. Chem. Soc. 1995, 117, 6414. |
| [20] | Johnson, L. K.; Mecking, S.; Brookhart, M. J. Am. Chem. Soc. 1996, 118, 267. |
| [21] | Fischer, K.; Jones, K.; Misbach, P.; Stabba, R.; Wilke G. Angew. Chem., Int. Ed. 1973, 12, 943. |
| [22] | Zou, C.; Si, G. F.; Chen, C. L. Nat. Commun. 2022, 13, 1954. |
| [23] | Wang, Y. Y.; Hu, X. Q.; Mu, H. L.; Xia, Y.; Chi, Y.; Jian, Z. B. Acta Chim. Sinica 2022, 80, 741. (in Chinese) |
| [23] | (王玉银, 胡小强, 穆红亮, 夏艳, 迟悦, 简忠保, 化学学报, 2022, 80, 741.) |
| [24] | Zhang, H.; Zou, C.; Zhao, H.; Cai, Z. G.; Chen, C. L. Angew. Chem., Int. Ed. 2021, 60, 17446. |
| [25] | Liang, T.; Goudari, S.; Chen, C. L. Nat. Commun. 2020, 11, 372. |
| [26] | Lin, F.; Mecking, S. Angew. Chem., Int. Ed. 2022, 134, e202203923. |
| [27] | Xin, B. S.; Sato, N.; Tanna, A.; Oishi, Y.; Konishi, Y.; Shimizu, F. J. Am. Chem. Soc. 2017, 139, 3611. |
| [28] | Zhang, Y.; Mu, H.; Pan, L.; Wang, X.; Li, Y. ACS Catal. 2018, 8, 5963. |
| [29] | Wang, X.; Zhang, Y.; Wang, F.; Pan, L.; Wang, B.; Li, Y. ACS Catal. 2021, 11, 2902. |
| [30] | Xiong, S.; Shoshani, M. M.; Zhang, X.; Spinney, H. A.; Nett, A. J.; Henderson, B. S.; Miller, T. F.; Agapie, T. J. Am. Chem. Soc. 2021, 143, 6516. |
| [31] | Baur, M.; Lin, F.; Morgen, T. O.; Odenwald, L.; Mecking, S. Science 2021, 374, 604. |
| [32] | Mecking, S.; Schnitte, M. Acc. Chem. Res. 2020, 53, 2738. |
| [33] | Du, W.; Zheng, H.; Li, Y.; Cheung, C. S.; Li, D.; Gao, H.; Deng, H.; Gao, H. Macromolecules 2022, 55, 3096. |
| [34] | Chen, S. Y.; Ren, B. H.; Li, S. H.; Song, Y. H.; Jiao, S.; Zou, C.; Chen, C. L.; Lu, X. B.; Liu, Y. Angew. Chem., Int. Ed. 2022, 61, e202204126. |
| [35] | Chen, Z.; Leatherman, M. D.; Daugulis, O.; Brookhart, M. J. Am. Chem. Soc. 2017, 139, 16013. |
| [36] | Chen, Z.; Brookhart, M. Acc. Chem. Res. 2018, 51, 1831. |
| [37] | Guo, L. H.; Dai, S. Y.; Sui, X. L.; Chen, C. L. ACS Catal. 2016, 6, 428. |
| [38] | Wang, F. Z.; Chen, C. L. Polym. Chem. 2019, 10, 2354. |
| [39] | Meinhard, D.; Wegner, M.; Kipiani, G.; Hearley, A.; Reuter, P.; Fischer, S.; Marti O.; Rieger, B. J. Am. Chem. Soc. 2007, 129, 9182. |
| [40] | Vaidya, T.; Klimovica, K.; LaPointe, A. M.; Keresztes, I.; Lobkovsky, E. B.; Daugulis, O.; Coates, G. W. J. Am. Chem. Soc. 2014, 136, 7213. |
| [41] | Wang, Z.; Liu, Q.; Solan, G. A.; Sun, W. H. Coord. Chem. Rev. 2017, 350, 68. |
| [42] | Li, J.; Peng, D.; Tan, C.; Chen, C. L. Angew. Chem., Int. Ed. 2023, 62, e202300359. |
| [43] | Tan, C.; Zou, C.; Chen, C. L. J. Am. Chem. Soc. 2022, 144, 2245. |
| [44] | Anderson Jr, W. C.; Rhinehart, J. L.; Tennyson, A. G.; Long, B. K. J. Am. Chem. Soc. 2016, 138, 774. |
| [45] | Doerr, A. M.; Curry, M. R.; Chapleski, R. C.; Burroughs, J. M.; Lander, E. K.; Roy, S.; Long, B. K. ACS Catal. 2021, 12, 73. |
| [46] | Long, B. K.; Eagan, J. M.; Coates, G. W. S. Angew. Chem., Int. Ed. 2016, 55, 7106. |
| [47] | Zhong, L.; Li, G.; Liang, G.; Gao, H.; Wu, Q. Macromolecules 2017, 50, 2675. |
| [48] | Kanai, Y.; Foro, S.; Plenio, H. Organometallics 2019, 38, 544. |
| [49] | Li, M.; Wang, X. B.; Luo, Y.; Chen, C. L. Angew. Chem., Int. Ed. 2017, 56, 11604. |
| [50] | Peng, D.; Chen, C. L. Angew. Chem., Int. Ed. 2021, 60, 22195. |
| [51] | Liao, Y. D.; Zhang, Y. X.; Cui, L.; Mu, H. L.; Jian, Z. B. Organometallics 2019, 38, 2075. |
| [52] | Pei, L.; Liu, F.; Liao, H.; Gao, J.; Zhong, L.; Gao, H.; Wu, Q. ACS Catal. 2018, 8, 1104. |
| [53] | Hu, X.; Zhang, Y.; Zhang, Y.; Jian, Z. ChemCatChem 2020, 12, 2497. |
| [54] | Gong, Y. F.; Li, S. K.; Gong, Q.; Zhang, S. J.; Liu, B. Y.; Dai, S. Y. Organometallics 2019, 38, 2919. |
| [55] | Fang, J.; Sui, X.; Li, Y.; Chen, C. Polym. Chem. 2018, 9, 4143. |
| [56] | Lu, Z.; Xu, X. W.; Luo, Y.; He, S. B.; Fan, W. G.; Dai, S. Y. ACS Catal. 2023, 13, 725. |
| [57] | Sun, Y.; Wang, Q.; Pan, Y.; Pang, W. M.; Zou, C.; Chen, M. Chin. J. Chem. 2022, 40, 2773. |
| [58] | Mahmood, Q.; Zeng, Y. N.; Yue, E. L.; Solan, G. A.; Liang, T. L.; Sun, W. H. Polym. Chem. 2017, 8, 6416. |
| [59] | Wang, X. X.; Fan, L. L.; Ma, Y. P.; Guo, C. Y.; Solan, G. A.; Sun, Y.; Sun, W. H. Polym. Chem. 2017, 8, 2785. |
| [60] | Chen, A.; Liao, D. H.; Chen, C. L. Chin. J. Chem. 2022, 40, 215. |
| [61] | Peng, D.; Xu, M. H.; Tan, C.; Chen, C. L. Macromolecules 2023, 56, 2388. |
| [62] | Ni1 (CCDC, 2257873); Ni3 (CCDC, 2257874). |
| [63] | Falivene, L.; Credendino, R.; Poater, A.; Petta, A.; Serra, L.; Oliva, R.; Scarano, V.; Cavallo, L. Organometallics 2016, 35, 2286. |
/
| 〈 |
|
〉 |