

Pd and Hg Atoms Co-doped HgPdAu23(PET)18 Nanocluster★
Received date: 2023-03-13
Online published: 2023-06-07
Supported by
Outstanding Doctoral Research Project of Yuncheng University(QZX-2023008); China Postdoctoral Foundation(2022M721551)
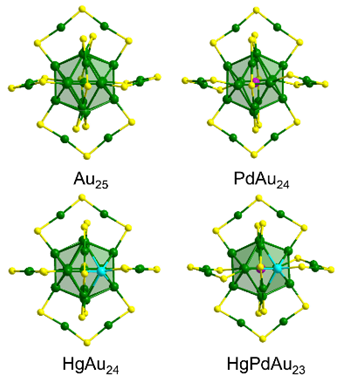
The introduction of heteroatoms into metal nanoclusters is an effective strategy to regulate the physical and chemical properties of clusters. Most of the doped clusters reported are binary metal nanoclusters via one metal heteroatom doping into a metal nanocluster, while the preparation and properties of ternary metal nanoclusters via two heteroatoms doping into a metal nanocluster are rarely studied. In this work, we report the Pd and Hg co-doped ternary metal nanocluster HgPdAu23(PET)18 (PET=2-phenylethanethiol) using the Au25(PET)18 nanocluster as an ideal template that can be viewed as a Au13 icosahedral core protected by the exterior 12 Au atoms as a shell. The structural framework of the HgPdAu23(PET)18 nanocluster is similar to that of its parent Au25(PET)18 nanocluster, determined by single-crystal X-ray crystallography and electrospray ionization mass spectroscopy (ESI-MS), which shows a 13-atom icosahedral core and a 12-atom shell capped by 18 thiolate ligands. It indicated that the doping strategy might destroy the total structure (core plus surface) of the parent nanocluster, which will offer an excited opportunity for the correlation of the structure with properties of the doped nanoclusters. More notably, the possible doping location of the Pd and Hg atoms in the HgPdAu23(PET)18 nanocluster can be determined by the combination of single-crystal X-ray crystallography and nuclear magnetic resonance (NMR) spectroscopy (i.e., 1H NMR and two-dimentional NMR). It is suggested that the Pd atom is possibly located on the center of the icosahedron of the HgPdAu23(PET)18 nanocluster, while the Hg atom is possibly located on the surface of the icosahedron of the HgPdAu23(PET)18 nanocluster. The electronic configuration of HgPdAu23(PET)18 nanocluster is distinct from those of the parent Au25(PET)18 and one-metal doped PdAu24(PET)18 and HgAu24(PET)18 nanoclusters, which can be implied by the X-ray photoelectron spectroscopy (XPS) and UV-Vis absorption spectroscopy. This study provides a new design rule for the two-metal-heteroatom doped nanoclusters.
Key words: nanocluster; heteroatoms; doping; structure
Yuying Zhang , Xiao Cai , Weigang Hu , Guangjun Li , Yan Zhu . Pd and Hg Atoms Co-doped HgPdAu23(PET)18 Nanocluster★[J]. Acta Chimica Sinica, 2023 , 81(7) : 703 -708 . DOI: 10.6023/A23030078
| [1] | Carrillo A. J.; Serra J. M. Catalysis 2021, 11, 741. |
| [2] | Senapati S.; Ahmad A.; Khan M. I.; Sastry M.; Kumar R. Small 2010, 1, 517. |
| [3] | Sugano Y.; Shiraishi Y.; Tsukamoto D.; Ichikawa S.; Tanaka S.; Hirai T. Angew. Chem., Int. Ed. 2013, 52, 5295. |
| [4] | Chao W.; Sheng P.; Chan R.; Sun S. Small 2010, 5, 567. |
| [5] | Li D. Q.; Zhang Z. Q.; Zang P. Y.; Ma Y. W.; Wu Q.; Yang L. J.; Chen Q.; Wang X. Z.; Hu Z. Acta Chim. Sinica 2016, 74, 587. (in Chinese) |
| [5] | (黎聃勤, 张志琦, 臧鹏远, 马延文, 吴强, 杨立军, 陈强, 王喜章, 胡征, 化学学报, 2016, 74, 587.) |
| [6] | Zhang Y. F.; Park S. J. J. Catal. 2017, 355, 1. |
| [7] | Jin Y. L.; Qin W.; Jiang Y.; Wang M.; Yao J. L.; Huang J.; Gu R. A. Acta Chim. Sinica 2008, 66, 2494. (in Chinese) |
| [7] | (金毅亮, 秦维, 蒋芸, 王梅, 姚建林, 黄洁, 顾仁敖, 化学学报, 2008, 66, 2494.) |
| [8] | Zhu C. Z.; Guo S. J.; Dong S. J. Adv. Mater. 2012, 24, 2326. |
| [9] | Fan X. Y.; Zhuang Q. C.; Wei G. Z.; Ke F. S.; Huang L.; Dong Q. F.; Sun S. G. Acta Chim. Sinica 2009, 67, 1547. (in Chinese) |
| [9] | (樊小勇, 庄全超, 魏国祯, 柯福生, 黄令, 董全峰, 孙世刚, 化学学报, 2009, 67, 1547.) |
| [10] | Yamazoe S.; Kurashige W.; Nobusada K.; Negishi Y.; Tsukuda T. J. Phys. Chem. C 2014, 118, 25284. |
| [11] | Negishi Y.; Munakata K.; Ohgake W.; Nobusada K. J. Phys. Chem. Lett. 2012, 3, 2209. |
| [12] | Cai X.; Saranya G.; Shen K. Q.; Chen M. Y.; Si R.; Ding W. P.; Zhu Y. Angew. Chem., Int. Ed. 2019, 58, 9964. |
| [13] | Wang S. X.; Song Y. B.; Jin S.; Liu X.; Zhang J.; Pei Y.; Meng X. M.; Chen M.; Li P.; Zhu M. Z. J. Am. Chem. Soc. 2015, 137, 4018. |
| [14] | Bhat S.; Baksi A.; Mudedla S. K.; Natarajan G.; Subramanian V.; Pradeep T. J. Phys. Chem. Lett. 2017, 8, 2787. |
| [15] | Walsh A. G.; Zhang P. J. Phys. Chem. Lett. 2021, 12, 257. |
| [16] | Sels A.; Salassa G.; Pollitt S.; Guglieri C.; Rupprechter G.; Barrabés N.; Bürgi T. J. Phys. Chem. C 2017, 121, 10919. |
| [17] | Nasaruddin R. R.; Hülsey M. J.; Xie J. P. Mol. Catal. 2022, 518, 112095. |
| [18] | Chen Q.; Qin Z. X.; Liu S.; Zhu M. C.; Li G.J. Chem. Phys. C 2021, 154, 164308. |
| [19] | Kang X.; Li Y. W.; Zhu M. Z.; Jin R. C. Chem. Soc. Rev. 2020, 49, 6443. |
| [20] | Qin Z. X.; Zhao D.; Zhao L.; Xiao Q.; Wu T. T.; Zhang J. W.; Wan C. Q.; Li G. Nanoscale Adv. 2019, 1, 2529. |
| [21] | Panapitiya G.; Wang H.; Chen Y. X.; Hussain E.; Jin R. C.; Lewis J. P. Phys. Chem. Chem. Phys. 2018, 20, 13747. |
| [22] | Kwak K.; Choi W.; Tang Q.; Kim M.; Lee Y.; Jiang D. E.; Lee D. Nat. Chem. 2017, 8, 14723. |
| [23] | Lu Y. Z.; Zhang C. M.; Li X. K.; Frojd A. R.; Xing W.; Clayborne A. Z.; Chen W. Nano Energy 2018, 50, 316. |
| [24] | Li S.; Alfonso D.; Nagarajan A. V.; House S. D.; Yang J. C.; Kauffman D. R.; Mpourmpakis G.; Jin R. C. ACS Catal. 2020, 10, 12011. |
| [25] | Yang S.; Wang S. X.; Jin S.; Chen S.; Sheng H. T.; Zhu M. Z. Nanoscale 2015, 7, 10005. |
| [26] | Suyama M.; Takano S.; Tsukuda T. J. Phys. Chem. C 2020, 124, 23923. |
| [27] | Bootharaju M. S.; Sinatraa L.; Bakr O. M. Nanoscale 2016, 8, 17333. |
| [28] | Fei W. W.; Antonello S.; Dainese T.; Dolmella A.; Lahtinen M.; Rissanen K.; Venzo A.; Maran F. J. Am. Chem. Soc. 2019, 141, 16033. |
| [29] | Zhu M. Z.; Lanni E.; Garg N.; Bier M. E.; Jin R. C. J. Am. Chem. Soc. 2008, 130, 1138. |
| [30] | Takano S.; Ito S.; Tsukuda T. J. Am. Chem. Soc. 2019, 141, 15994. |
/
| 〈 |
|
〉 |