

Synthesis of Pyrrolo[3,2-d]pyrimidin-4-ones via Cascade Alkyne−isocyanide [3+2] Cycloaddition/Boulton-Katritzky Rearrangement/Ring Expansion Process★
Received date: 2023-04-26
Online published: 2023-06-07
Supported by
National Natural Science Foundation of China(22001093); National Natural Science Foundation of China(21971087); Guangdong Basic and Applied Basic Research Foundation(2022A1515010200)
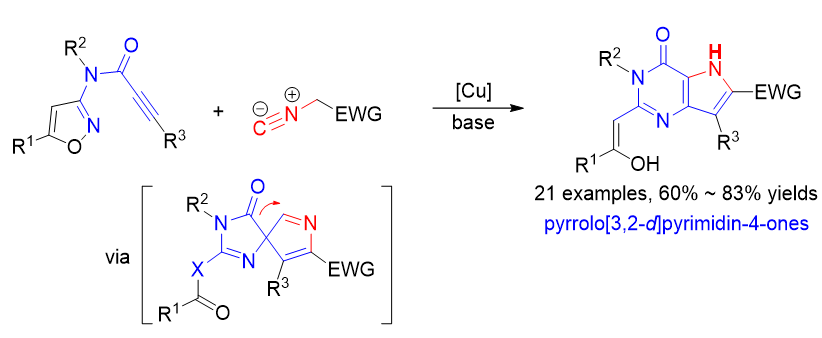
Pyrrolo[3,2-d]pyrimidin-4-ones are valuable structural motifs in many bioactive compounds and pharmaceuticals. Such structures have received extensive attentions from synthetic community. Two general strategies have been developed for the formation of the fused pyrimidine-pyrrole structure: one is to construct the pyrimidine ring from substituted pyrroles, and the other is to construct the pyrrole ring from 5,6-functionalized pyrimidines. However, these methods have generally required multiple synthetic steps and the use of starting materials with uncommon functional groups, and also suffered with other drawbacks such as harsh reaction conditions and limited substrate scope. Thus, it is highly desirable to develop facile and practical approaches for the construction of structural diversified pyrrolo[3,2-d]pyrimidin-4-ones. Very recently, we have developed an alkyne-isocyanide [3+2] cycloaddition/Boulton-Katritzky rearrangement/ring expansion reaction for the synthesis of 9-deazaguanines from 1,2,4-oxadiazole-derived propiolamides with isocyanides. Different from traditional Boulton-Katritzky rearrangement (BKR), which is to form stable five-membered rings, the method provides a facile access to fused heterocycles via forming an unstable BKR spirocyclic intermediate and followed by a spontaneous ring expansion via acyl migration. In this work, to further expand the scope of this method, the [3+2] cycloaddition/BKR-ring expansion reactions of isoxazole-derived propiolamides with isocyanides were developed. The reactions were performed with CuI as the catalyst and Cs2CO3 as the base in toluene/N,N-dimethylformamide (DMF) (1∶3, V∶V) at room temperature for the alkyne-isocyanide [3+2] cycloaddition and then at 110 ℃ for the BKR-ring expansion process. A variety of isoxazole- derived propiolamides and isocyanides were well tolerated in the reactions and afforded the desired pyrrolo[3,2- d]pyrimidin-4-one products in satisfactory yields. The control experiments were performed to elucidate the reaction process: copper catalyst is used only for the alkyne-isocyanide [3+2] cycloaddition, but no necessary for the base-promoted BKR-ring expansion process. Compared to the traditional methods for such skeletons, the approach features readily available starting materials, broad substrate scope, short steps, and structural diversification.
Jianghao Luo , Haowen Ma , Jiehao Zhang , Wei Zhou , Qian Cai . Synthesis of Pyrrolo[3,2-d]pyrimidin-4-ones via Cascade Alkyne−isocyanide [3+2] Cycloaddition/Boulton-Katritzky Rearrangement/Ring Expansion Process★[J]. Acta Chimica Sinica, 2023 , 81(8) : 898 -904 . DOI: 10.6023/A23040164
| [1] | (a) Bantia S.; Miller P. J.; Parker C. D.; Ananth S. L.; Horn L. L.; Kilpatrick J. M.; Morris P. E.; Hutchison T. L.; Montgomery J. A.; Sandhu J. S.; Int. Immunopharmacol. 2001, 1, 1199; |
| [1] | (b) Theoclitou M.-E.; Aquila, B.; Block, M. H.; Brassil, P. J.; Castriotta, L.; Code, E.; Collins, M. P.; Davies, A. M.; Deegan, T.; Ezhuthachan, J.; Filla, S.; Freed, E.; Hu, H.; Huszar, D.; Jayaraman, M.; Lawson, D.; Lewis, P. M.; Nadella, M. V. P.; Oza, V.; Padmanilayam, M.; Pontz, T.; Ronco, L.; Russell, D.; Whitston, D.; Zheng, X. J. Med. Chem. 2011, 54, 6734; |
| [1] | (c) Laufersweiler M. C.; Wang Y.; Soper D. L.; Suchanek M. K.; Fancher A. N.; Lu W.; Wang R. L.; Oppong K. A.; Ellis C. D.; Baize M. W.; O'Neil S. V.; Wos J. A.; Demuth T. P. Bioorg. Med. Chem. Lett. 2005, 15, 4322. |
| [2] | (a) Semeraro T.; Lossani A.; Botta M.; Ghiron C.; Alvarez R.; Manetti F.; Mugnaini C.; Valensin S.; Focher F.; Corelli F.; J. Med. Chem. 2006, 49, 6037; |
| [2] | (b) Rodrigues M. V. N.; Barbosa, A. F.; da Silva, J. F.; dos Santos, D. A.; Vanzolini, K. L.; de Moraes, M. C.; Corrêa, A. G.; Cass, Q. B. Bioorg. Med. Chem. 2016, 24, 226; |
| [2] | (c) Tian C.; Wang, M.; Fang, F.; Zhang, Z.; Wang, X.; Liu, J. Eur. J. Med. Chem. 2017, 138, 630; |
| [2] | (d) Gangjee A.; Li, W.; Yang, J.; Kisliuk, R. L. J. Med. Chem. 2008, 51, 68; |
| [2] | (e) Huang L.; Li, H.; Li, L.; Niu, L.; Seupel, R.; Wu, C.; Cheng, W.; Chen, C.; Ding, B.; Brennan, P. E.; Yang, S. J. Med. Chem. 2019, 62, 4526; |
| [2] | (f) Zeng S.; Xie H.; Zeng L.-L.; Lu X.; Zhao X.; Zhang G.-C.; Tu Z.-C.; Xu H.-J.; Yang L.; Zhang X.-Q.; Hu W. Bioorg. Med. Chem. 2013, 21, 1749. |
| [3] | (a) Imai K.-I. Chem. Pharm. Bull. 1964, 12, 1030 |
| [3] | (b) Taylor, E. C.; Young, W. B.; Ward, C. C. Tetrahedron Lett. 1993, 34, 4595;. |
| [3] | (c) Taylor, E. C.; Young, W. B. J. Org. Chem. 1995, 60, 7947;. |
| [3] | (d) Elliott, A. J.; Montgomery, J. A.; Walsh, D. A. Tetrahedron Lett. 1996, 37, 4339;. |
| [3] | (e) Furneaux, R. H.; Tyler, P. C. J. Org. Chem. 1999, 64, 8411;. |
| [3] | (f) Liu, M.-C.; Luo, M.-Z.; Mozdziesz, D. E.; Sartorelli, A. C. Synth. Commun. 2002, 32, 3797;. |
| [3] | (g) Elliott, A. J.; Morris, P. E.; Petty, Jr. S. L.; Williams, C. H. J. Org. Chem. 1997, 62, 8071;. |
| [3] | (h) Kwong, C. D.; Elliot, A. J.; Montgomery, J. A. J. Labelled Cpd. Radiopharm. 1998, 41, 879;. |
| [3] | (i) Shih H.; Cottam H. B.; Carson D. A. Chem. Pharm. Bull. 2002, 50, 364. |
| [4] | (a) Boulton A. J.; Katritzky A. R.; Hamid A. M. J. Chem. Soc. C 1967, 2005; |
| [4] | (b) Afridi, A. S.; Katritzky, A. R.; Ramsden, C. A. J. Chem. Soc., Perkin Trans. 1 1976, 315;. |
| [4] | (c) For a book: Comprehensive Heterocyclic Chemistry II, Eds.: Katritzky, A. R.; Rees, C. W.; Scriven, E. F. V., Elsevier, Amsterdam, 1996, Vols. 1-9. |
| [5] | For selected reviews and examples of rearrangement with isoxazoles and 1,2,4-oxadiazoles, see: (a) Pace, A.; Pierro, P.; Buscemi, S.; Vivona, N.; Barone, G. J. Org. Chem. 2009, 74, 351; |
| [5] | (b) Martorana, A.; Piccionello, A. P.; Buscemi, S.; Giorgi, G.; Pace, A. Org. Biomol. Chem. 2011, 9, 491;. |
| [5] | (c) Martorana, A.; Pace, A.; Buscemi, S.; Piccionello, A. P. Org. Lett. 2012, 14, 3240;. |
| [5] | (d) Hu, F.; Szostak, M. Adv. Synth. Catal. 2015, 357, 2583;. |
| [5] | (e) Jones R. C. F.; Chatterley A.; Marty R.; Owton W. M.; Elsegood M. R. J. Chem. Commun. 2015, 51, 1112. |
| [6] | For selected reviews on ring expansion reactions, see: (a) Lu, B.-L.; Dai, L.; Shi, M. Chem. Soc. Rev. 2012, 41, 3318; |
| [6] | (b) Mack, D. J.; Njardarson, J. T. ACS Catal. 2013, 3, 272; |
| [6] | (c) Biletskyi, B.; Colonna, P.; Masson, K.; Parrain, J.-L.; Commeiras, L.; Chouraqui, G. Chem. Soc. Rev. 2021, 50, 7513; |
| [6] | (d) Li, D.; Zang, W.; Bird, M. J.; Hyland, C. J. T.; Shi, M. Chem. Rev. 2021, 121, 8685; |
| [6] | (e) Nanda, T.; Fastheem, M.; Linda, A.; Pati, B. V.; Banjare, S. K.; Biswal, P.; Ravikumar, P. C. ACS Catal. 2022, 12, 13247; |
| [6] | (f) Ye J. Chin. J. Org. Chem. 2021, 41, 1755. |
| [7] | For selected examples on ring expansion reactions, see: (a) Luzung, M. R.; Markham, J. P.; Toste, F. D. J. Am. Chem. Soc. 2004, 126, 10858; |
| [7] | (b) Gorin, D. J.; Davis, N. R.; Toste, F. D. J. Am. Chem. Soc. 2005, 127, 11260; |
| [7] | (c) Chen, G.-Q.; Zhang, X. N.; Wei, Y.; Tang, X.-Y.; Shi, M. Angew. Chem., Int. Ed. 2014, 53, 8492; |
| [7] | (d) Pan, D.; Wei, Y.; Shi, M. Org. Lett. 2016, 18, 3930; |
| [7] | (e) Chen, G.-Q.; Fang, W.; Wei, Y.; Tang, X.-Y.; Shi, M. Chem. Sci. 2016, 7, 4318; |
| [7] | (f) Pan, D.; Wei, Y.; Shi, M. Org. Lett. 2017, 19, 3584; |
| [7] | (g) Wu, Q.-F.; Zheng, C.; You, S.-L. Angew. Chem., Int. Ed. 2012, 51, 1680; |
| [7] | (h) Zhuo, C.-X.; Wu, Q.-F.; Zhao, Q.; Xu, Q.-L.; You, S.-L. J. Am. Chem. Soc. 2013, 135, 8169; |
| [7] | (i) Zhuo, C.-X.; Cheng, Q.; Liu, W.-B.; Zhao, Q.; You, S.-L. Angew. Chem., Int. Ed. 2015, 54, 8475; |
| [7] | (j) Wu, Q.; Zheng, C.; Zhuo, C.-X.; You, S.-L. Chem. Sci. 2016, 7, 4453; |
| [7] | (k) Wang, Y.; Zheng, C.; You, S.-L. Angew. Chem., Int. Ed. 2017, 56, 15093; |
| [7] | (l) Zheng, C.; You, S.-L. Acc. Chem. Res. 2020, 53, 974; |
| [7] | (m) George, J.; Kim, H. Y.; Oh, K. Adv. Synth. Catal. 2016, 358, 3714; |
| [7] | (n) George, J.; Kim, H. Y.; Oh, K. Org. Lett. 2017, 19, 628; |
| [7] | (o) Ramu, G.; Ambala, S.; Nanubolu, J. B.; Babu, B. N. RSC Adv. 2019, 9, 35068; |
| [7] | (p) Ramu, G.; Tangella, Y.; Ambala, S.; Babu, B. N. J. Org. Chem. 2020, 85, 5370; |
| [7] | (q) Moisan, L.; Wagner, M.; Comesse, S.; Doris, E. Tetrahedron Lett. 2006, 47, 9093; |
| [7] | (r) Pesquet, A.; Da?ch, L. Van Hijfte, J. Org. Chem. 2006, 71, 5303; |
| [7] | (s) Shang, S.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. Angew. Chem., Int. Ed. 2014, 53, 6216; |
| [7] | (t) Guo, X.; Xing, Q.; Lei, K.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. Adv. Synth. Catal. 2017, 359, 4393; |
| [7] | (u) Mamedov, V. A.; Mamedova, V. L.; Qu, Z.-W.; Zhu, H.; Galimullina, V. R.; Korshin, D. E.; Khikmatova, G. Z.; Litvinov, I. A.; Latypov, S. K.; Sinyashin, O. G.; Grimme, S. J. Org. Chem. 2021, 86, 13514; |
| [7] | (v) Mandal, S.; Pramanik, A. J. Org. Chem. 2022, 87, 9282; |
| [7] | (w) Zhu W.-K.; Xu L.-W. Chin. J. Org. Chem. 2023, 43, 362. (in Chinese) |
| [7] | ( 祝炜柯, 徐利文, 有机化学, 2023, 43, 362.) |
| [8] | For recent reviews on heterocyclic dearomatization: (a) Roche, S. P.; Porco, J. A. Angew. Chem., Int. Ed. 2011, 50, 4068; |
| [8] | (b) Donohoe, T. J.; Pullin, R. D. C. Chem. Commun. 2012, 48, 11924; |
| [8] | (c) Ding, Q.; Zhou, X.; Fan, R. Org. Biomol. Chem. 2014, 12, 4807; |
| [8] | (d) Zhuo, C.-X.; Zheng, C.; You, S.-L. Acc.Chem. Res. 2014, 47, 2558; |
| [8] | (e) Liang, X.-W.; Zheng, C.; You, S.-L. Chem. Eur. J. 2016, 22, 11918; |
| [8] | (f) Sun, W.; Li, G.; Hong, L.; Wang, R. Org. Biomol. Chem. 2016, 14, 2164; |
| [8] | (g) Zheng, C.; You, S.-L. Chem 2016, 1, 830; |
| [8] | (h) Wu W.-T.; Zhang L.-M.; You S.-L. Acta Chim. Sinica 2017, 75, 419. (in Chinese) |
| [8] | ( 吴文挺, 张立明, 游书力, 化学学报, 2017, 75, 419.) |
| [9] | Luo J.; Ma H.; Wu K.; Ao Y.; Zhou W.; Cai Q. Org. Lett. 2023, 25, 2123. |
| [10] | For selected reviews on alkyne-isocyanide [3+2] cycloaddition, see: (a) Gulevich, A. V.; Zhdanko, A. G.; Orru, R. V. A.; Nenajdenko, V. G. Chem. Rev. 2010, 110, 5235; |
| [10] | (b) Boyarskiy, V. P.; Bokach, N. A.; Luzyanin, K. V.; Kukushkin, V. Y. Chem. Rev. 2015, 115, 2698; |
| [10] | (c) Giustiniano, M.; Basso, A.; Mercalli, V.; Massarotti, A.; Novellino, E.; Tron, G. C.; Zhu, J. Chem. Soc. Rev. 2017, 46, 1295; |
| [10] | (d) Luo J.; Chen G.-S.; Chen S.-J.; Li Z.-D.; Liu Y.-L. Chem. Eur. J. 2021, 27, 6598. |
| [11] | For selected examples, see: (a) Kamijo, S.; Kanazawa, C.; Yamamoto, Y. J. Am. Chem. Soc. 2005, 127, 9260; |
| [11] | (b) Larionov, O. V.; de Meijere, A. Angew. Chem., Int. Ed. 2005, 44, 5664; |
| [11] | (c) Gao, M.; He, C.; Chen, H.; Bai, R.; Cheng, B.; Lei, A. Angew. Chem., Int. Ed. 2013, 52, 6958; |
| [11] | (d) Liu, J.; Fang, Z.; Zhang, Q.; Liu, Q.; Bi, X. Angew. Chem., Int. Ed. 2013, 52, 6953; |
| [11] | (e) Qi, X.; Zhang, H.; Shao, A.; Zhu, L.; Xu, T.; Gao, M.; Liu, C.; Lan, Y. ACS Catal. 2015, 5, 6640; |
| [11] | (f) Xiao, P.; Yuan, H.; Liu, J.; Zheng, Y.; Bi, X.; Zhang, J. ACS Catal. 2015, 5, 6177; |
| [11] | (g) Dong, J.; Bao, L.; Hu, Z.; Ma, S.; Zhou, X.; Hao, M.; Li, N.; Xu, X. Org. Lett. 2018, 20, 1244; |
| [11] | (h) He, X.-L.; Zhao, H.-R.; Song, X.; Jiang, B.; Du, W.; Chen, Y.-C.; ACS Catal. 2019, 9, 4374; |
| [11] | (i) Zheng, S.-C.; Wang, Q.; Zhu, J. Angew. Chem., Int. Ed. 2019, 58, 1494; |
| [11] | (j) Liu, J.-Q.; Chen, X.; Shatskiy, A.; K?rk?s, M. D.; Wang, X.-S. J. Org. Chem. 2019, 84, 8998; |
| [11] | (k) Wang Y.; Zhou Y.; Song Q. Chem. Commun. 2020, 56, 6106. |
| [12] | Bird C. W. Tetrahedron 1985, 41, 1409. |
| [13] | CCDC 2258385 (3j) contains the supplementary crystallographic data for this paper. |
/
| 〈 |
|
〉 |