

Advances in Catalytic Asymmetric Reactions Involving o-Hydroxyphenyl Substituted p-Quinone Methides★
Received date: 2023-04-30
Online published: 2023-06-15
Supported by
National Natural Science Foundation of China(22125104); National Natural Science Foundation of China(21831007); National Natural Science Foundation of China(22101103); Natural Science Foundation of Jiangsu Province(BK20201018); Science and Technology Plan of Xuzhou City(KC21021)
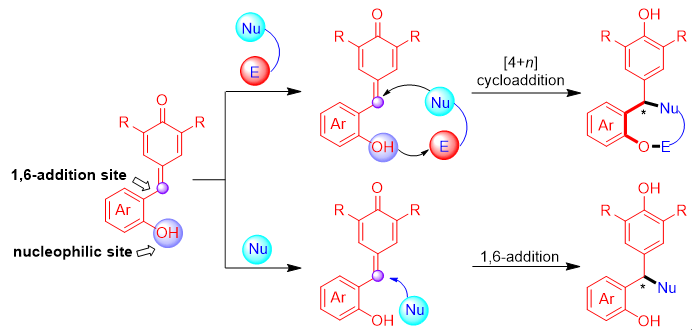
o-Hydroxyphenyl substituted p-quinone methides (p-QMs) belong to a class of p-QMs with unique advantages. They not only maintain the high reactivity of p-QMs, but also have more reactive and activation sites owing to the introduction of hydroxyl group. Therefore, o-hydroxyphenyl substituted p-QMs have wide applications in synthetic and medicinal chemistry. The catalytic asymmetric 1,6-conjugate addition and [4+n] cycloaddition of o-hydroxyphenyl substituted p-QMs have developed very rapidly in recent years, which have become efficient strategies for the synthesis of chiral oxygen-containing heterocycles and arylmethanes with potential bioactivity. This review summarizes the catalytic asymmetric reactions involving o-hydroxyphenyl substituted p-QMs and points out the remaining challenges in this research area, which will open a new window for the design of new type of o-hydroxyphenyl substituted p-QMs and their involved catalytic asymmetric reactions.
Shuang Yang , Ningyi Wang , Qingqing Hang , Yuchen Zhang , Feng Shi . Advances in Catalytic Asymmetric Reactions Involving o-Hydroxyphenyl Substituted p-Quinone Methides★[J]. Acta Chimica Sinica, 2023 , 81(7) : 793 -808 . DOI: 10.6023/A23040192
| [1] | For some examples: (a) Nonaka G.; Kawahara O.; Nishioka I.. Chem. Pharm. Bull. 1982, 30, 4277. |
| [1] | (b) Sawadjoon S.; Kittakoop P.; Kirtikara K.; Vichai V.; Tanticharoen M.; Thebtaranonth Y. J. Org. Chem. 2002, 67, 5470. |
| [1] | (c) Zhang X. F.; Wang H. M.; Song Y. L.; Nie L. H.; Wang L. F.; Liu B.; Shen P. P.; Liu Y. Bioorg. Med. Chem. Lett. 2006, 16, 949. |
| [2] | For some examples: (a) Batra J. K.; Kang G. J.; Jurd L.; Hamel E.; Biochem. Pharmac. 1988, 37, 2595. |
| [2] | (b) Kasibhatla S.; Gourdeau H.; Meerovitch K.; Drewe J.; Reddy S.; Qiu L.; Zhang H.; Bergeron F.; Bouffard D.; Yang Q.; Herich J.; Lamothe S.; Cai S. X.; Tseng B. Mol. Cancer Ther. 2004, 3, 11. |
| [2] | (c) De Campos M. P.; Filho V. C.; Da Silva R. Z.; Yunes R. A.; Zacchino S.; Juarez S.; Bella Cruz R. C.; Bella Cruz A. Biol. Pharm. Bull. 2005, 28, 1527. |
| [3] | For some examples: (a) Hucke O.; Gelb M. H.; Verlinde C. L. M. J.; Buckner F. S. J. Med. Chem. 2005, 48, 5415. |
| [3] | (b) Shagufta; Srivastava A. K.; Sharma R.; Mishra R.; Balapure A. K.; Murthy P. S. R.; Panda G. Bioorg. Med. Chem. 2006, 14, 1497. |
| [3] | (c) Wood P. M.; Woo L. W. L.; Labrosse J. R.; Trusselle M. N.; Abbate S.; Longhi G.; Castiglioni E.; Lebon F.; Purohit A.; Reed M. J.; Potter B. V. L. J. Med. Chem. 2008, 51, 4226. |
| [4] | For some reviews: (a) Shang Y.; Xiao J.; Wang Y.; Peng Y. Acta Chim. Sinica 2021, 79, 1303. (in Chinese) |
| [4] | (尚阳, 肖检, 王雅雯, 彭羽, 化学学报, 2021, 79, 1303.) |
| [4] | (b) Zhang L.; Xiao J.; Wang Y.; Peng Y. Acta Chim. Sinica 2022, 80, 1152. (in Chinese) |
| [4] | (张崃, 肖检, 王雅雯, 彭羽, 化学学报, 2022, 80, 1152.) |
| [5] | For some early reviews: (a) Turner A. B. Q. Rev. Chem. Soc. 1964, 18, 347. |
| [5] | (b) Peter M. G. Angew. Chem. Int. Ed. 1989, 28, 555. |
| [5] | (c) Itoh T. Prog. Polym. Sci. 2001, 26, 1019. |
| [5] | (d) Toteva M. M.; Richard J. P. Adv. Phys. Org. Chem. 2011, 45, 39. |
| [6] | For some selected reviews: (a) Parra A.; Tortosa M. ChemCatChem 2015, 7, 1524. |
| [6] | (b) Caruana L.; Fochi M.; Bernardi L. Molecules 2015, 20, 11733. |
| [6] | (c) Li W., Xu X.; Zhang P.; Li P. Chem. Asian J. 2018, 13, 2350. |
| [6] | (d) Lima C. G. S.; Pauli F. P.; Costa D. C. S.; Souza A. S.; Forezi L. S. M.; Ferreira V. F.; de Carvalho da Silva F. Eur. J. Org. Chem. 2020, 18, 2650. |
| [6] | (e) Wang J. Y.; Hao W. J.; Tu S. J.; Jiang B. Org. Chem. Front. 2020, 7, 1743. |
| [6] | (f) Singh G.; Pandey R.; Pankhade Y. A.; Fatma S.; Anand R. V. Chem. Rec. 2021, 21, 4150. |
| [6] | (g) Hussain Y.; Tamanna; Sharma M.; Kumar A.; Chauhan P. Org. Chem. Front. 2022, 9, 572. |
| [7] | Zhao K.; Zhi Y.; Shu T.; Valkonen A.; Rissanen K.; Enders D. Angew. Chem. Int. Ed. 2016, 55, 12104. |
| [8] | Zhang L.; Zhou X.; Li P.; Liu Z.; Liu Y.; Suna Y.; Li W. RSC Adv. 2017, 7, 39216. |
| [9] | Duan C.; Ye L.; Xu W.; Li X.; Chen F.; Zhao Z.; Li X. Chin. Chem. Lett. 2018, 29, 1273. |
| [10] | (a) Annunziata F.; Pinna C.; Dallavalle S.; Tamborini L.; Pinto A. Int. J. Mol. Sci. 2020, 21, 4618. |
| [10] | (b) Cheke R. S.; Patel H. M.; Patil V. M.; Ansari I. A.; Ambhore J. P.; Shinde S. D.; Kadri A.; Snoussi M.; Adnan M.; Kharkar P. S.; Pasupuleti V. R.; Deshmukh P. K. Antibiotics 2022, 11, 566. |
| [10] | (c) Ratre P.; Kulkarni S.; Das S.; Liang C.; Mishra P. K.; Thareja S. Med. Oncol. 2023, 40, 41. |
| [11] | (a) Kanchana U.S.; Diana E. J.; Mathew T. V.; Anilkumar G. Appl. Organomet. Chem. 2020, 34, e5983. |
| [11] | (b) Moreira N. M.; Martelli L. S. R.; Corrêa A. G. Beilstein J. Org. Chem. 2021, 17, 1952. |
| [12] | Zhang Z. P.; Chen L.; Li X.; Cheng J. P. J. Org. Chem. 2018, 83, 2714. |
| [13] | Xiang M.; Li C. Y.; Song X. J.; Zou Y.; Huang Z. C.; Li X.; Tian F.; Wang L. X. Chem. Commun. 2020, 56, 14825. |
| [14] | Zhao M.; Li F.; Cheng Y.; Wang Y.; Zhou Z. Chin. J. Org. Chem. 2021, 41, 4039. (in Chinese) |
| [14] | (赵敏, 李霏, 程益政, 王有名, 周正洪, 有机化学, 2021, 41, 4039.) |
| [15] | For some selected reviews: (a) Akiyama T. Chem. Rev. 2007, 107, 5744. |
| [15] | (b) Terada M. Synthesis 2010, 12, 1929. |
| [15] | (c) Yu J.; Shi F.; Gong L. Z. Acc. Chem. Res. 2011, 44, 1156. |
| [15] | (d) Parmar D.; Sugiono E.; Raja S.; Rueping M. Chem. Rev. 2014, 114, 9047. |
| [15] | (e) Xia Z. L.; Xu-Xu Q. F.; Zheng C.; You S. L. Chem. Soc. Rev. 2020, 49, 286. |
| [15] | For some recent examples: (f) Wang J. Y.; Sun M.; Yu X. Y.; Zhang Y. C.; Tan W.; Shi F. Chin. J. Chem. 2021, 39, 2163. |
| [15] | (g) Sheng F. T.; Yang S.; Wu S. F.; Zhang Y. C.; Shi F. Chin. J. Chem. 2022, 40, 2151. |
| [15] | (h) Shi Y. C.; Yan X. Y.; Wu P.; Jiang S.; Xu R.; Tan W.; Shi F. Chin. J. Chem. 2023, 41, 27. |
| [16] | Zhang L.; Liu Y.; Liu K.; Liu Z.; He N.; Li W. Org. Biomol. Chem. 2017, 15, 8743. |
| [17] | Zhang Z. P.; Xie K. X.; Yang C.; Li M.; Li X. J. Org. Chem. 2018, 83, 364. |
| [18] | Jiang X. L.; Wu S. F.; Wang J. R.; Mei G. J.; Shi F. Adv. Synth. Catal. 2018, 360, 4225. |
| [19] | Wu S. F.; Tu M. S.; Hang Q. Q.; Zhang S.; Ding H.; Zhang Y. C.; Shi F. Org. Biomol. Chem. 2020, 18, 5388. |
| [20] | Yang G. H.; Zhao Q.; Zhang Z. P.; Zheng H. L.; Chen L.; Li X. J. Org. Chem. 2019, 84, 7883. |
| [21] | Li H. H.; Meng Y. N.; Chen C. M.; Wang Y. Q.; Zhang Z. X.; Xu Z.; Zhou B.; Ye L. W. Sci. China Chem. 2023, DOI:10.1007/s11426-022-1536-9. |
| [22] | For some recent examples: (a) Tan J. P.; Li K.; Shen B.; Zhuang C.; Liu Z.; Xiao K.; Yu P.; Yi B.; Ren X; Wang T. Nat. Commun. 2022, 13, 357. |
| [22] | (b) Chen Y.; He J.; Zhuang C.; Liu Z.; Xiao K.; Su Z.; Ren X.; Wang T. Angew. Chem. Int. Ed. 2022, 61, e202207334. |
| [22] | (c) Hu H. L.; Ren X.; He J.; Zhu L.; Fang S.; Su Z.; Wang T. Sci. China Chem. 2022, 65, 2500. |
| [22] | (d) Wu J. H.; Tan J. P.; Zheng J. Y.; He J.; Song Z.; Su Z.; Wang T. Angew. Chem. Int. Ed. 2023, 62, e202215720. |
| [23] | Tan J. P.; Zhang H.; Jiang Z.; Chen Y.; Ren X.; Jiang C.; Wang T. Adv. Synth. Catal. 2020, 362, 1058. |
| [24] | Roy S.; Pradhan S.; Kumar K.; Chatterjee I. Org. Chem. Front. 2020, 7, 1388. |
| [25] | For early reviews: (a) Dalko P. I.; Moisan L. Angew. Chem. Int. Ed. 2001, 40, 3726. |
| [25] | (b) Dalko P. I. Enantioselective Organocatalysis: Reactions and Experimental Procedures, Wiley-VCH, Weinheim, Germany, 2007. |
| [25] | (c) List B. Chem. Rev. 2007, 107, 5413. |
| [25] | (d) MacMillan D. W. C. Nature 2008, 455, 304. |
| [25] | For recent reviews: (e) Wang Y. B.; Tan B. Acc. Chem. Res. 2018, 51, 534. |
| [25] | (f) Metrano A. J.; Miller S. J. Acc. Chem. Res. 2019, 52, 199 |
| [25] | (g) Zhang Y. C.; Jiang F.; Shi F. Acc. Chem. Res. 2020, 53, 425. |
| [25] | (h) Zhang H. H.; Shi F. Acc. Chem. Res. 2022, 55, 2562. |
| [25] | (i) Cheng J. K.; Xiang S. H.; Tan B. Acc. Chem. Res. 2022, 55, 2920. |
| [25] | (j) Yang J.; Pan B. W.; Chen L.; Zhou Y.; Liu X. L. Chem. Synth. 2023, 3, 7. |
| [25] | (k) Wang Z. H.; Sun T. J.; Zhang Y. P.; You Y.; Zhao J. Q.; Yin J. Q.; Yuan W. C. Chem. Synth. 2023, 3, 12. |
| [26] | For some recent examples: (a) Han Z.; Zhuang H.; Tang L.; Zang Y.; Guo W.; Huang H.; Sun J. Org. Lett. 2022, 24, 4246. |
| [26] | (b) Hang Q. Q.; Wu S. F.; Yang S.; Wang X.; Zhong Z.; Zhang Y. C.; Shi F. Sci. China Chem. 2022, 65, 1929. |
| [26] | (c) Zhu Z.; Shi F. Chin. J. Org. Chem. 2022, 42, 2996. |
| [26] | (d) Wu P.; Yan X. Y.; Jiang S.; Lu Y. N.; Tan W.; Shi F. Chem. Synth. 2023, 3, 6. |
| [26] | (e) Liu Y. W.; Chen Y. H.; Cheng J. K.; Xiang S. H.; Tan B. Chem. Synth. 2023, 3, 11. |
| [26] | (f) Zhu G. Y.; Zhou J. J.; Liu L. G.; Li X.; Zhu X. Q.; Lu X.; Zhou J. M.; Ye L. W. Angew. Chem. Int. Ed. 2022, 61, e202204603. |
| [26] | (g) Wang Z. S.; Zhu L. J.; Li C. T.; Liu B. Y.; Hong X.; Ye L. W. Angew. Chem. Int. Ed. 2022, 61, e202201436. |
| [26] | (h) Chen Y.; He J.; Zhuang C.; Liu Z.; Xiao K.; Su Z.; Ren X.; Wang T. Angew. Chem. Int. Ed. 2022, 61, e202207334. |
| [26] | (i) Zhu L.; Peng H.; Guo Y.; Che J.; Wu J. H. Su Z.; Wang T. Angew. Chem. Int. Ed. 2022, 61, e202202467. |
| [27] | Zhao M. X.; Xiang J.; Zhao Z. Q.; Zhao X. L.; Shi M. Org. Biomol. Chem. 2020, 18, 1637. |
| [28] | For some recent reviews: (a) Luo J.; Chen G. S.; Chen S. J.; Li Z. D.; Liu Y. L. Chem. Eur. J. 2021, 27, 6598. |
| [28] | (b) Laviós A.; Sanz-Marco A.; Vila C.; Blay G.; Pedro J. R. Eur. J. Org. Chem. 2021, 2021, 2268. |
| [28] | (c) Wang J.; Li D.; Li J.; Zhu Q. Org. Biomol. Chem. 2021, 19, 6730. |
| [29] | Yang J.; Ming S.; Yao G.; Yu H.; Du Y.; Gong J. Org. Chem. Front. 2022, 9, 2759. |
| [30] | For some selected early reviews: (a) Harmata M. Chem. Commun. 2010, 46, 8904. |
| [30] | (b) Harmata M. Chem. Commun. 2010, 46, 8886. |
| [30] | (c) Lohse A. G.; Hsung R. P. Chem. Eur. J. 2011, 17, 3812. |
| [31] | For a recent highlight: (a) Tan W.; Shi F. Chem. Synth. 2022, 2, 11. |
| [31] | For a recent review: (b) Tan W.; Zhang J. Y.; Gao C. H.; Shi F. Sci. China: Chem. 2023, 66, 966. |
| [32] | Li W.; Yuan H.; Liu Z.; Zhang Z.; Cheng Y.; Li P. Adv. Synth. Catal. 2018, 360, 2460. |
| [33] | Liu Q.; Li S.; Chen X. Y.; Rissanen K.; Enders D. Org. Lett. 2018, 20, 3622. |
| [34] | Jiang F.; Yuan F. R.; Jin L. W.; Mei G. J.; Shi F. ACS Catal. 2018, 8, 10234. |
| [35] | (a) Wu Y.; Zhou X.; Xiao W.; Chen J. Chin. J. Org. Chem. 2020, 40, 3760. (in Chinese) |
| [35] | (吴雅莉, 周雪松, 肖文精, 陈加荣, 有机化学, 2020, 40, 3760.) |
| [35] | (b) Sarkar T.; Talukdar K.; Das B. K.; Shah T. A.; Debnath B.; Punniyamurthy T. Org. Biomol. Chem. 2021, 19, 3776. |
| [35] | (c) Niu B.; Wei Y.; Shi M. Org. Chem. Front. 2021, 8, 3475. |
| [35] | (d) Du Q.; Zhang L.; Gao F.; Wang L.; Zhang W. Chin. J. Org. Chem. 2022, 42, 3240. (in Chinese) |
| [35] | (杜青锋, 张璐, 高峰, 王乐, 张万斌, 有机化学, 2022, 42, 3240.) |
| [36] | Sun M.; Ma C.; Zhou S. J.; Lou S. F.; Xiao J.; Jiao Y.; Shi F. Angew. Chem. Int. Ed. 2019, 58, 8703. |
| [37] | Wang H.; Yang S.; Zhang Y.; Shi F. Chin. J. Org. Chem. 2023, 43, 974. (in Chinese) |
| [37] | (王海清, 杨爽, 张宇辰, 石枫, 有机化学, 2023, 43, 974.) |
| [38] | For some reviews: (a) Mei G. J.; Shi F. J. Org. Chem. 2017, 82, 7695. |
| [38] | (b) Petrini M. Adv. Synth. Catal. 2020, 362, 1214. |
| [38] | (c) Zhang H. H.; Shi F. Chin. J. Org. Chem. 2022, 42, 3351. (in Chinese) |
| [38] | (张洪浩, 石枫, 有机化学, 2022, 42, 3351.) |
| [39] | For some reviews: (a) Chen D. F.; Han Z. Y.; Zhou X. L.; Gong L. Z. Acc. Chem. Res. 2014, 47, 2365. |
| [39] | (b) Wang P. S.; Chen D. F.; Gong L. Z. Top. Curr. Chem. 2020, 378, 9. |
| [39] | (c) Wang P. S.; Gong L. Z. Acc. Chem. Res. 2020, 53, 2841. |
| [39] | (d) Zhang J.; Gao J.; Feng J.; Lu T.; Du D. Chin. J. Org. Chem. 2021, 41, 3792. (in Chinese) |
| [39] | (张建明, 高健, 冯捷, 陆涛, 杜鼎, 有机化学, 2021, 41, 3792.) |
| [39] | (e) Chen D. F.; Gong L. Z. J. Am. Chem. Soc. 2022, 144, 2415. |
| [39] | (f) Xiang X.; He Z.; Dong X. Chin. J. Org. Chem. 2023, 43, 791. (in Chinese) |
| [39] | (向勋, 何照林, 董秀琴, 有机化学, 2023, 43, 791.) |
| [40] | Balanna K.; Barik S.; Shee S.; Gonnade R. G.; Biju A. T. Chem. Sci. 2022, 13, 11513. |
| [41] | For some selected examples: (a) Goodman C. G.; Johnson J. S. J. Am. Chem. Soc. 2014, 136, 14698. |
| [41] | (b) Goodman C. G.; Walker M. M., Johnson J. S. J. Am. Chem. Soc. 2015, 137, 122. |
| [41] | (c) Chen X.; Fong J. Z. M.; Xu J.; Mou C.; Lu Y.; Yang S.; Song B. A.; Chi Y. R. J. Am. Chem. Soc. 2016, 138, 7212. |
| [41] | See also: (d) Liu B.; Song R.; Xu J.; Majhi P. K.; Yang X.; Yang S.; Jin Z.; Chi Y. R. Org. Lett. 2020, 22, 3335. |
| [42] | For some reviews: (a) Nevagi R. J.; Dighe S. N.; Dighe S. N. Eur. J. Med. Chem. 2015, 97, 561. |
| [42] | (b) Radadiya A.; Shah A. Eur. J. Med. Chem. 2015, 97, 356. |
| [43] | Zielke K.; Ková? O.; Winter M.; Pospí?il J.; Waser M. Chem. Eur. J. 2019, 25, 8163. |
| [44] | For some reviews: (a) Ma S. Chem. Rev. 2005, 105, 2829. |
| [44] | (b) Cowen B. J.; Miller S. J. Chem. Soc. Rev. 2009, 38, 3102. |
| [44] | (c) Yu S.; Ma S. Angew. Chem., Int. Ed. 2012, 51, 3074. |
| [44] | (d) Pei C. K.; Shi M. Chem. Eur. J. 2012, 18, 6712. |
| [44] | (e) Ye J.; Ma S. Acc. Chem. Res. 2014, 47, 989. |
| [44] | (f) Yang L.; Ma J. Acta Chim. Sinica 2016, 74, 130. (in Chinese) |
| [44] | (杨丽军, 马军安, 化学学报, 2016, 74, 130.). |
| [44] | (g) Li G.; Huo X.; Jiang X.; Zhang W. Chem. Soc. Rev. 2020, 49, 2060. |
| [45] | Lu H.; Zhang H. X.; Tan C. Y.; Liu J. Y.; Wei H.; Xu P. F. J. Org. Chem. 2019, 84, 10292. |
| [46] | Tan J. P.; Yu P.; Wu J. H.; Chen Y.; Pan J.; Jiang C.; Ren X.; Zhang H. S.; Wang T. Org. Lett. 2019, 21, 7298. |
| [47] | (a) Dalpozzo R. Org. Chem. Front. 2017, 4, 2063. |
| [47] | (b) Dalpozzo R. Adv. Synth. Catal. 2017, 359, 1772. |
| [47] | (c) Cao Z. Y.; Zhou F.; Zhou J. Acc. Chem. Res. 2018, 51, 1443. |
| [47] | (d) Boddy A. J.; Bull J. A. Org. Chem. Front. 2021, 8, 1026. |
| [48] | Wu Y. C.; Cui B. D.; Long Y.; Han W. Y.; Wan N. W.; Yuan W. C.; Chen Y. Z. Adv. Synth. Catal. 2021, 363, 1702. |
| [49] | Zhang L.; Yuan H.; Lin W.; Cheng Y.; Li P.; Li W. Org. Lett. 2018, 20, 4970. |
| [50] | For some reviews, see: (a) Schindler C. S.; Jacobsen E. N. Science 2013, 340, 1052. |
| [50] | (b) Bihani M.; Zhao J. C. G. Adv. Synth. Catal. 2017, 359, 534. |
| [50] | (c) Lin L.; Feng X. Chem. Eur. J. 2017, 23, 6464. |
| [50] | (d) Zhan G.; Du W.; Chen Y. C. Chem. Soc. Rev. 2017, 46, 1675. |
| [50] | (e) Krautwald S.; Carreira E. M. J. Am. Chem. Soc. 2017, 139, 5627. |
| [50] | (f) Beletskaya I. P.; Na?jera C.; Yus M. Chem. Rev. 2018, 118, 5080. |
| [50] | (g) Nájera C.; Foubelo F.; Sansano J. M.; Yus M. Org. Biomol. Chem. 2020, 18, 1232. |
| [50] | (h) Huo X.; Li G.; Wang X.; Zhang W. Angew. Chem. Int. Ed. 2022, 61, e202210086. |
| [51] | For some reviews, see: (a) Nambo M.; Crudden C. M. ACS Catal. 2015, 5, 4734. |
| [51] | (b) Kshatriya R.; Jejurkar V. P.; Saha S. Eur. J. Org. Chem. 2019, 2019, 3818. |
| [51] | (c) Liu X.; Wu X.; Zhang L.; Lin X.; Huang D. Synthesis 2020, 52, 2311. |
| [52] | Huang G. B.; Huang W. H.; Guo J.; Xu D. L.; Qu X. C.; Zhai P. H.; Zheng X. H.; Weng J.; Lu G. Adv. Synth. Catal. 2019, 361, 1241. |
| [53] | Wang J. R.; Jiang X. L.; Hang Q. Q.; Zhang S.; Mei G. J.; Shi F. J. Org. Chem. 2019, 84, 7829. |
| [54] | Cheng Y.; Fang Z.; Jia Y.; Lu Z.; Li W.; Li P. RSC Adv. 2019, 9, 24212. |
| [55] | Zhang R. L.; Liu B.; Qiu K. X.; Li H. T.; Zhang H. N.; Shen B. C.; Sun Z. W. Org. Lett. 2023, 25, 1711. |
| [56] | For selected reports, see: (a) Li P.; Chan S. H.; Chan A. S. C.; Kwong F. Y. Org. Biomol. Chem. 2011, 9, 7997. |
| [56] | (b) Maity R.; Gharui C.; Sil A. K.; Pan S. C. Org. Lett. 2017, 19, 662. |
| [56] | (c) Maity R.; Pan S. C. Org. Biomol. Chem. 2018, 16, 1598. |
| [56] | (d) Sahoo S. C.; Maity R.; Pan S. C. ACS Omega 2019, 4, 2792. |
| [56] | (e) Liu Y. Y.; Mo Y. R.; Dong X. D.; Chen L.; Ye L.; Li X. Y.; Zhao Z. G.; Li X. F. Tetrahedron 2019, 75, 2466. |
| [57] | Zhou J.; Bai L. J.; Liang G. J.; Xu Q. G.; Zhou L. P.; Zhou H. Org. Biomol. Chem. 2020, 18, 2641. |
| [58] | For some selected reviews: (a) Tang W.; Zhang X. Chem. Rev. 2003, 103, 3029. |
| [58] | (b) Horsman G. P.; Zechel D. L. Chem. Rev. 2017, 117, 5704. |
| [59] | For some selected reviews: (a) Li Z.; Duan W. Chin. J. Org. Chem. 2016, 36, 1805. (in Chinese) |
| [59] | (李振, 段伟良, 有机化学, 2016, 36, 1805.) |
| [59] | (b) Zhu R. Y.; Liao K.; Yu J. S.; Zhou J. Acta Chim. Sinica 2020, 78, 193. (in Chinese) |
| [59] | (朱仁义, 廖奎, 余金生, 周剑, 化学学报, 2020, 78, 193.) |
| [59] | (c) Zhang F.; Luan Y.; Ye M. Chin. J. Org. Chem. 2021, 41, 3880. (in Chinese) |
| [59] | (张凤萍, 栾玉新, 叶萌春, 有机化学, 2021, 41, 3880.) |
| [60] | Chen Y.; Yu Z.; Jiang Z.; Tan J. P.; Wu J. H.; Lan Y.; Ren X.; Wang T. ACS Catal. 2021, 11, 14168. |
/
| 〈 |
|
〉 |