

Synthesis and Application of Organic Hypervalent Bromine Reagents
Received date: 2023-04-30
Online published: 2023-06-26
Supported by
National Natural Science Foundation of China(22271069); National Natural Science Foundation of China(21871067); Guangdong Basic and Applied Basic Research Foundation(2021A1515010190); Guangdong Basic and Applied Basic Research Foundation(2023A1515012457)
In recent decades, with the rapid development of organic synthetic chemistry, organic hypervalent halogen reagents have drawn considerable research interest on the synthesis and application of organic hypervalent bromine reagents. Compared with traditional organic hypervalent iodine reagents, organic hypervalent bromine reagents have stronger oxidation capacity and reactivity, thus enabling their important application potential in organic synthesis. On the basis of different substituents of organic hypervalent bromine reagents, the diaryl-λ3-bromanes, dialkyl-λ3-bromanes, alkenyl-λ3-bromanes, alkyne-λ3-bromanes and heteroatomic-λ3-bromanes are summarized and discussed successively. The synthetic methods of different types of hypervalent bromine reagents and their applications in organic reactions are also reviewed.
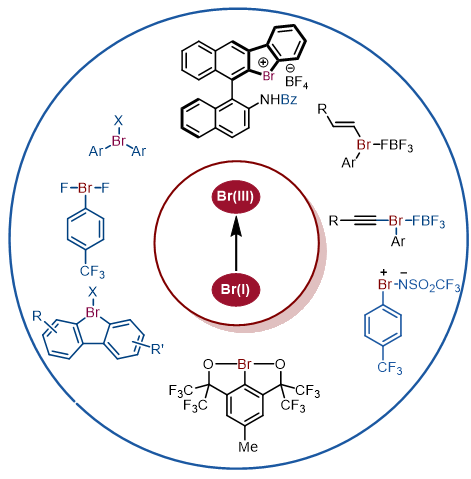
Shaoyan Gan , Shengyu Zhong , Liting Wang , Lei Shi . Synthesis and Application of Organic Hypervalent Bromine Reagents[J]. Acta Chimica Sinica, 2023 , 81(8) : 1030 -1042 . DOI: 10.6023/A23040173
| [1] | Musher J. I. Angew. Chem., Int. Ed. 1969, 8, 54. |
| [2] | Jensen W. B. J. Chem. Educ. 2006, 83, 1751. |
| [3] | Pauling L. J. Am. Chem. Soc. 1932, 54, 3570. |
| [4] | Zhu D.; Yao Y.; Zhao R.; Liu Y.; Shi L. Chem.-Eur. J. 2018, 24, 4805. |
| [5] | Taylor M. T.; Nelson J. E.; Suero M. G.; Gaunt M. J. Nature 2018, 562, 563. |
| [6] | Banik S. M.; Medley J. W.; Jacobsen E. N. Science 2016, 353, 6294. |
| [7] | (a) Yoshimura A.; Zhdankin V. V. Chem. Rev. 2016, 116, 3328. |
| [7] | (b) Zhao R.; Shi L. Angew. Chem., Int. Ed. 2020, 59, 12282. |
| [7] | (c) Zhdankin V. V.; Stang P. J. Chem. Rev. 2008, 108, 5299. |
| [7] | (d) Song A. R.; Zhang C. Acta Chim. Sinica 2015, 73, 1002. (in Chinese) |
| [7] | ( 宋爱茹, 张弛, 化学学报, 2015, 73, 1002.) |
| [7] | (e) Duan Y. N.; Jiang S. N.; Han Y. C.; Sun B.; Zhang C. Chin. J. Org. Chem. 2016, 36, 1973. (in Chinese) |
| [7] | ( 段亚南, 姜山, 韩永超, 孙博, 张弛, 有机化学, 2016, 36, 1973.) |
| [7] | (f) Cai Q.; Ma H. W. Acta Chim. Sinica 2019, 77, 213. (in Chinese) |
| [7] | ( 蔡倩, 马浩文, 化学学报, 2019, 77, 213.) |
| [8] | Pauling L. J. Am. Chem. Soc. 1932, 54, 3570. |
| [9] | CRC Handbook of Chemistry and Physics, Ed.: Lide, D. R., CRC Press, Boca Raton, FL, 1992, pp. 1-2408. |
| [10] | (a) Ochiai M. Synlett 2009, 159. |
| [10] | (b) Farooq U.; Shah A.-H. A.; Wirth T. Angew. Chem., Int. Ed. 2009, 48, 101. |
| [10] | (c) Miyamoto K. PATAI’S Chemistry of Functional Groups, Ed.: Rappoport, Z., Wiley, Chichester, 2018, pp. 1-25. |
| [11] | Leneau P. Ann. Chim. Phys. 1906, 9, 241. |
| [12] | Sandin R. B.; Hay A. S. J. Am. Chem. Soc. 1952, 74, 274. |
| [13] | Miyamoto K.; Saito M.; Tsuji S.; Takagi T.; Shiro M.; Uchiyama M.; Ochiai M. J. Am. Chem. Soc. 2021, 143, 9327. |
| [14] | (a) Nesmeyanov A. N.; Vanchikov A. N.; Lisichkina I. N.; Lazarev V. V.; Tolstaya T. P. Dokl. Akad. Nauk SSSR 1980, 255, 1136. |
| [14] | (b) Nesmeyanov A. N.; Vanchikov A. N.; Lisichkina I. N.; Khruscheva N. S.; Tolstaya T. P. Dokl. Akad. Nauk SSSR 1980, 254, 652. |
| [15] | Holleman A. F.; Wiberg E. Inorganic Chemistry, Academic Press, San Diego, 2001. |
| [16] | Frohn H. J.; Giesen M. J. Fluorine Chem. 1984, 24, 9. |
| [17] | Lanzi M.; Dherbassy Q.; Wencel-Delord J. Angew. Chem., Int. Ed. 2021, 60, 14852. |
| [18] | (a) Grushin V. V. Chem. Soc. Rev. 2000, 29, 315. |
| [18] | (b) Chatterjee N.; Goswami A. Eur. J. Org. Chem. 2017, 2017, 3023. |
| [19] | Lanzi M.; Ali Abdine R. A.; De Abreu M.; Wencel-Delord J. Org. Lett. 2021, 23, 9047. |
| [20] | Zhang Y.; Han J.; Liu Z. RSC Adv. 2015, 5, 25485. |
| [21] | Yoshida Y.; Ishikawa S.; Mino T.; Sakamoto M. Chem. Commun. 2021, 57, 2519. |
| [22] | Yoshida Y.; Mino T.; Sakamoto M. ACS Catal. 2021, 11, 13028. |
| [23] | Olah G. A.; DeMember J. R. J. Am. Chem. Soc. 1969, 91, 2113. |
| [24] | Slebocka-Tilk H.; Ball R. G.; Brown R. S. J. Am. Chem. Soc. 1985, 107, 4504. |
| [25] | Prakash G. K. S.; Bruce M. R.; Olah G. A. J. Org. Chem. 1985, 50, 2405. |
| [26] | Ochiai M.; Nishi Y.; Mori T.; Tada N.; Suefuji T.; Frohn H. J. J. Am. Chem. Soc. 2005, 127, 10460. |
| [27] | Miyamoto K.; Shiro M.; Ochiai M. Angew. Chem., Int. Ed. 2009, 48, 8931. |
| [28] | Ochiai M. J. Organomet. Chem. 2000, 611, 494. |
| [29] | Ochiai M.; Okubo T.; Miyamoto K. J. Am. Chem. Soc. 2011, 133, 3342. |
| [30] | Ochiai M.; Tada N.; Okada T.; Sota A.; Miyamoto K. J. Am. Chem. Soc. 2008, 130, 2118. |
| [31] | Ochiai M.; Nishi Y.; Goto S.; Shiro M.; Frohn H. J. J. Am. Chem. Soc. 2003, 125, 15304. |
| [32] | (a) Kitamura T.; Stang P. J. J. Org. Chem. 1988, 53, 4105. |
| [32] | (b) Stang P.J.; Surber B. W.; Chen Z.-C.; Roberts K. A.; Anderson A. G. J. Am. Chem. Soc. 1987, 109, 228. |
| [33] | Ochiai M.; Nishi Y.; Goto S.; Shiro M.; Frohn H. J. J. Am. Chem. Soc. 2003, 125, 15304. |
| [34] | (a) Donaldson R. E.; Fuchs P. L. J. Am. Chem. Soc. 1981, 103, 2108. |
| [34] | (b) Saddler J. C.; Fuchs P. L. J. Am. Chem. Soc. 1981, 103, 2112. |
| [34] | (c) Paquette L. A.; Lin H. S.; Gunn B. P.; Coghlan M. J. J. Am. Chem. Soc. 1988, 110, 5818. |
| [35] | Ochiai M.; Nishi Y.; Goto S.; Frohn H. J. Angew. Chem., Int. Ed. 2005, 44, 406. |
| [36] | Ochiai M.; Tada N. Chem. Commun. 2005, 5083. |
| [37] | (a) Evans D. A.; Faul M. M.; Bilodeau M. T. J. Am. Chem. Soc. 1994, 116, 2742. |
| [37] | (b) Li Z.; Quan R. W.; Jacobsen E. N. J. Am. Chem. Soc. 1995, 117, 5889. |
| [38] | Ochiai M.; Kaneaki T.; Tada N.; Miyamoto K.; Chuman H.; Shiro M.; Hayashi S.; Nakanishi W. J. Am. Chem. Soc. 2007, 129, 12938. |
| [39] | Hoque M. M.; Miyamoto K.; Tada N.; Shiro M.; Ochiai M. Org. Lett. 2011, 13, 5428. |
| [40] | Miyamoto K.; Ota T.; Hoque M. M.; Ochiai M. Org. Biomol. Chem. 2015, 13, 2129. |
| [41] | Espino C. G.; Bois J. D. In Modern Rhodium-Catalyzed Organic Reactions, Ed.: Evans, P. A., Wiley-VCH, Weinheim, 2005, p. 379 and references therein. |
| [42] | Ochiai M.; Miyamoto K.; Kaneaki T.; Hayashi S.; Nakanishi W.; Science 2011, 332, 448. |
| [43] | Miyamoto K.; Shiro M.; Ochiai M. Angew. Chem., Int. Ed. 2009, 48, 8931. |
| [44] | Ochiai M.; Yoshimura A.; Mori T.; Nishi Y.; Hirobe M. J. Am. Chem. Soc. 2008, 130, 3742. |
| [45] | Ochiai M.; Yoshimura A.; Miyamoto K.; Hayashi S.; Nakanishi W. J. Am. Chem. Soc. 2010, 132, 9236. |
| [46] | Ochiai M.; Tada N.; Murai K.; Goto S.; Shiro M. J. Am. Chem. Soc. 2006, 128, 9608. |
| [47] | Miyamoto K.; Iwasaki S.; Doi R.; Ota T.; Kawano Y.; Yamashita J.; Sakai Y.; Tada N.; Ochiai M.; Hayashi S.; Nakanishi W.; Uchiyama M. J. Org. Chem. 2016, 81, 3188. |
| [48] | Nguyen T. T.; Martin J. C. J. Am. Chem. Soc. 1980, 102, 7382. |
| [49] | Sokolovs I.; Mohebbati N.; Francke R.; Suna E. Angew. Chem., Int. Ed. 2021, 60, 15832. |
/
| 〈 |
|
〉 |