

Polycyclic Aromatic Hydrocarbon Molecule with Ultra-low LUMO/HOMO Energy Levels★
Received date: 2023-04-18
Online published: 2023-07-07
Supported by
National Natural Science Foundation of China(22075273); National Natural Science Foundation of China(22275029); Science and Technology Development Program of Jilin Province(20220204097YY)
Organic small molecules of low LUMO/HOMO (lowest unoccupied molecular orbital/highest occupied molecular orbital) energy levels are in shortage in terms of both variety and quantity. Their design and synthesis have important scientific and application value. The traditional strategy for designing organic small molecules of ultra-low LUMO/HOMO energy levels is to introduce multiple cyano groups into the molecules. In this work, a polycyclic aromatic hydrocarbon molecule containing four boron and nitrogen coordination bonds and two imide groups is designed and synthesized, without involving any cyano groups. The cyclic voltammetry examination with 0.1 mol•L−1 tetrabutylammonium hexafluorophosphate solution in dichloromethane as the electrolyte solution and ferrocene as the internal standard indicates that the molecule has a low LUMO and HOMO energy level of -4.77 eV and -6.39 eV, respectively. These values are the lowest for the reported fused-ring small molecules with boron and nitrogen coordination bonds and comparable to those of the reported organic small molecules with cyano groups. The density functional theory calculation results at the B3LYP/6-31G(d,p) theoretical level show that the molecule has a curved configuration and its conjugate skeleton has a dihedral angle of 23.6°. Both LUMO and HOMO are uniformly delocalized on the linear benzoid skeleton. It shows obvious near-infrared absorption in both solution and thin film state, with maximum absorption at 768 nm in film. The molecule can be used as a p-type dopant. After its blend doping, the electrical conductivity of the film of a typical p-type polymer P3HT is improved by 3 orders of magnitude. The doping behavior is also confirmed by UV-Vis absorption spectroscopy and electron paramagnetic resonance spectroscopy. This work develops a new strategy to achieve ultra-low LUMO energy level for organic small molecules without using cyano groups.
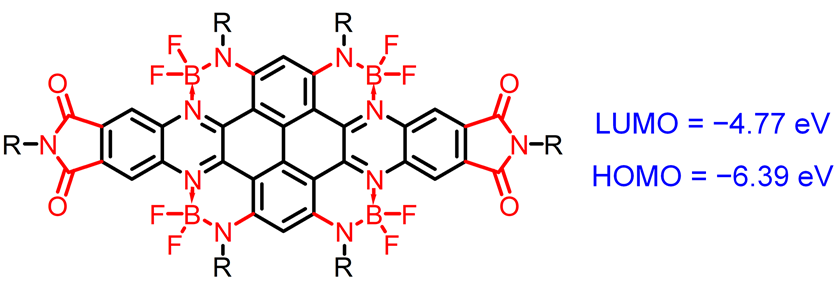
Key words: energy level; boron nitrogen coordination bond; imide; p-doping; fused-ring molecule
Rui Liu , Bin Meng , Junli Hu , Jun Liu . Polycyclic Aromatic Hydrocarbon Molecule with Ultra-low LUMO/HOMO Energy Levels★[J]. Acta Chimica Sinica, 2023 , 81(10) : 1295 -1300 . DOI: 10.6023/A23040142
| [1] | Wang, C. L.; Dong, H. L.; Jiang, L.; Hu, W. P. Chem. Soc. Rev. 2018, 47, 422. |
| [2] | Quinn, J. T. E.; Zhu, J. X.; Li, X.; Wang, J. L.; Li, Y. N. J. Mater. Chem. C 2017, 5, 8654. |
| [3] | Lakshminarayana, A. N.; Ong, A.; Chi, C. Y. J. Mater. Chem. C 2018, 6, 3551. |
| [4] | Takimiya, K.; Osaka, I.; Nakano, M. Chem. Mater. 2014, 26, 587. |
| [5] | Li, X.-J.; Li, Y.-F. Acta Polym. Sin. 2022, 53, 995 (in Chinese). |
| [5] | (李骁骏, 李永舫, 高分子学报, 2022, 53, 995.) |
| [6] | Li, T.-F.; Zhan, X.-W. Acta Chim. Sinica 2021, 79, 257 (in Chinese). |
| [6] | (李腾飞, 占肖卫, 化学学报, 2021, 79, 257.) |
| [7] | Gao, X. K.; Hu, Y. B. J. Mater. Chem. C 2014, 2, 3099. |
| [8] | Zaumseil, L.; Sirringhaus, H. Chem. Rev. 2007, 107, 1296. |
| [9] | Zhao, Y.; Guo, Y. L.; Liu, Y. Q. Adv. Mater. 2013, 25, 5372. |
| [10] | Bruder, I.; Watanabe, S.; Qu, J. Q.; Müller, I. B.; Kopecek, R.; Hwang, J.; Weis, J.; Langer, N. Org. Electron. 2010, 11, 589. |
| [11] | Wang, J.; Wang, Y. Z.; Li, K. C.; Dai, X.; Zhang, L. Y.; Wang, H. Adv. Mater. 2022, 34, 2106624. |
| [12] | Bunz, U. H. F.; Engelhart, J. U.; Lindner, B. D.; Schaffroth, M. Angew. Chem. Int. Ed. 2013, 52, 2. |
| [13] | Lin, G.-B.; Luo, T.; Yuan, L.-B.; Liang, W.-J.; Xu, H. Prog. Chem. 2017, 29, 1316 (in Chinese). |
| [13] | (林高波, 罗婷, 袁铝兵, 梁文杰, 徐海, 化学进展, 2017, 29, 1316.) |
| [14] | Lee, M. H. Adv. Electron. Mater. 2019, 5, 1900573. |
| [15] | Li, S.-Y.; Zhu, C.-Y.-J.; Luo, Y.-H.; Zhang, Y.-R.; Teng, H.-M.; Wang, Z.-R.; Zhen, Y.-G. Acta Chim. Sinica 2022, 80, 1600 (in Chinese). |
| [15] | (李善武, 朱陈宇杰, 罗尹豪, 张亚茹, 滕汉明, 王宗瑞, 甄永刚, 化学学报, 2022, 80, 1600.) |
| [16] | Chen, H.; Moser, M.; Wang, S. H.; Jellett, C.; Thorley, K.; Harrison, G. T.; Jiao, X. C.; Xiao, M. F.; Purushothaman, B.; Alsufyani, M.; Bristow, H.; Wolf, S. D.; Gasparini, N.; Wadsworth, A.; McNeill, C. R.; Sirringhaus, H.; Fabiano, S.; McCulloch, I. J. Am. Chem. Soc. 2021, 143, 260. |
| [17] | Yassar, A.; Demanze, F.; Jaafari, A.; Idrissi, M. E.; Coupry, C. Adv. Funct. Mater. 2002, 12, 699. |
| [18] | Liu, Z. T.; Zhang, G. X.; Cai, Z. X.; Chen, X.; Luo, H. W.; Li, Y. H.; Wang, J. G.; Zhang, D. Q. Adv. Mater. 2014, 26, 6965. |
| [19] | Bao, Z. N.; Lovinger, A. J.; Brown, J. J. Am. Chem. Soc. 1998, 120, 207. |
| [20] | Peltier, J. D.; Heinrich, B.; Donnio, B.; Jeannin, O.; Rault-Berthelot, J.; Jacques, E.; Poriel, C. J. Mater. Chem. C 2018, 6, 13197. |
| [21] | Liang, N. N.; Meng, D.; Wang, Z. H. Acc. Chem. Res. 2021, 54, 961. |
| [22] | Li, C.; Lin, Z.; Li, Y.; Wang, Z. H. Chem. Rec. 2016, 16, 873. |
| [23] | Tu, K-H.; Wang, Y.; Kiyota, Y.; Iwahashi, T.; Ouchi, Y.; Mori, T.; Michinobu, T. Org. Electron. 2020, 87, 105978. |
| [24] | Maitrot, M.; Guilfaud, G.; Boudjema, B.; André, J. J.; Simon, J. J. Appl. Phys. 1986, 60, 2396. |
| [25] | Aragay, G.; Frontera, A.; Lloveras, V.; Vidal-Gancedo, J.; Ballester, P. J. Am. Chem. Soc. 2013, 135, 2620. |
| [26] | Gao, Z. Q.; Mi, B. X.; Xu, G. Z.; Wan, Y. Q.; Gong, M. L.; Cheahab, K. W.; Chen, C. H. Chem. Commun. 2008, 117. |
| [27] | Kivala, M.; Boudon, C.; Gisselbrecht, J.-P.; Enko, B.; Seiler, P.; Müller, I. B.; Langer, N.; Jarowski, P. D.; Gescheidt, G.; Diederich, F. Chem. Eur. J. 2009, 15, 4111. |
| [28] | Koech, P. K.; Padmaperuma, A. B.; Wang, L.; Swensen, J. S.; Polikarpov, E.; Darsell, J. T.; Rainbolt, J. E.; Gaspar, D. J. Chem. Mater. 2010, 22, 3926. |
| [29] | Chang, J. J.; Ye, Q.; Huang, K.-W.; Zhang, J.; Chen, Z.-K.; Wu, J. S.; Chi, C. Y. Org. Lett. 2012, 14, 2964. |
| [30] | Gao, J.; Xiao, C. Y.; Jiang, W.; Wang, Z. H. Org. Lett. 2014, 16, 394. |
| [31] | Dou, C. D.; Long, X. J.; Ding, Z. C.; Xie, Z. Y.; Liu, J.; Wang, L. X. Angew. Chem. Int. Ed. 2016, 55, 1436. |
| [32] | Min, Y.; Dou, C. D.; Tian, H. K.; Geng, Y. H.; Liu, J.; Wang, L. X. Angew. Chem. Int. Ed. 2018, 57, 2000. |
| [33] | Liu, R.; Hu, J. L.; Min, Y.; Liu, J. Org. Chem. Front. 2023, 10, 923. |
| [34] | Min, Y.; Cao, X.; Tian, H. K.; Liu, J.; Wang, L. X. Chem. Eur. J. 2021, 27, 2065. |
| [35] | Min, Y.; Dou, C. D.; Tian, H. K.; Liu, J.; Wang, L. X. Chem. Eur. J. 2021, 27, 4364. |
| [36] | Min, Y.; Dou, C. D.; Liu, D.; Dong, H. L.; Liu, J. J. Am. Chem. Soc. 2019, 141, 17015. |
| [37] | Min, Y.; Dong, C. S.; Tian, H. K.; Liu, J.; Wang, L. X. ACS Appl. Mater. Interfaces 2021, 13, 33321. |
| [38] | Wu, Z. H.; Huang, Z. T.; Guo, R. X.; Sun, C. L.; Chen, L. C.; Sun, B.; Shi, Z. F.; Shao, X. F.; Li, H. Y.; Zhang, H. L. Angew. Chem. Int. Ed. 2017, 56, 13031. |
| [39] | Sun, Y. M.; Di, C. A.; Xu, W.; Zhu, D. B. Adv. Electron. Mater. 2019, 5, 1800825. |
| [40] | Lee, S.; Kim, S.; Pathak, A.; Tripathi, A.; Qiao, T.; Lee, Y.; Lee, H.; Woo, H. Y. Macromol. Res. 2020, 28, 531. |
| [41] | Liu, J.; Ye, G.; Zee, B. V. D.; Dong, J. J.; Qiu, X. K.; Liu, Y. R.; Portale, G.; Chiechi, R. C.; Koster, L. J. A. Adv. Mater. 2018, 30, 1804290. |
| [42] | Bredas, J. L.; Street, G. B. Acc. Chem. Res. 1985, 18, 309. |
| [43] | Xuan, Y.; Liu, X.; Desbief, S.; Leclère, P.; Fahlman, M.; Lazzaroni, R.; Berggren, M.; Cornil, J.; Emin, D.; Crispin, X. Phys. Rev. B 2010, 83, 115454. |
| [44] | Shin, Y.; Massetti, M.; Komber, H.; Biskup, T.; Nava, D.; Lanzani, G.; Caironi, M.; Sommer, M. Adv. Electron. Mater. 2018, 4, 1700581. |
| [45] | Kiefer, D.; Giovannitti, A.; Sun, H.; Biskup, T.; Hofmann, A.; Koopmans, M.; Cendra, C.; Weber, S.; Koster, L. J. A.; Olsson, E.; Rivnay, J.; Fabiano, S.; McCulloch, I.; Mu?ller, C. ACS Energy Lett. 2018, 3, 278. |
/
| 〈 |
|
〉 |