

Layered Uranyl Phosphonate as A Dual-response Luminescence Thermometer★
Received date: 2023-05-19
Online published: 2023-07-18
Supported by
National Natural Science Foundation of China(21731003); National Natural Science Foundation of China(21976014); National Natural Science Foundation of China(22276013); National Key Research Program(2021YFB3501501)
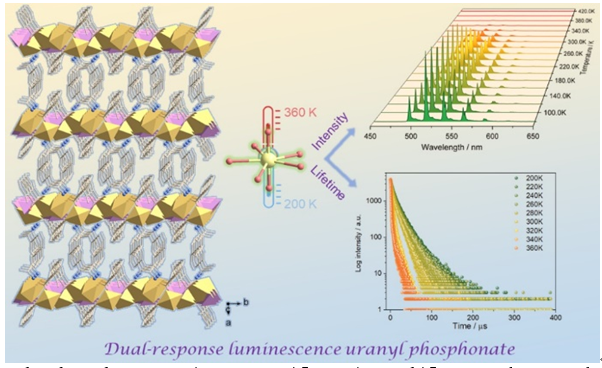
Luminescent uranyl phosphonate coordination polymers have been used for temperature sensing but have not yet been used for dual-response luminescence thermometers. Herein we report a luminescent uranyl phosphonate based on 2-(phosphonomethyl)benzoic acid (2-pmbH3), namely, (α-C8H12N)[UO2(2-pmb)] (1). This compound crystallizes in monoclinic space group C2/c and shows a layered structure. Within the layer, the uranyl ions are doubly bridged by O—P—O and O—C—O unis forming chains. The equivalent chains are cross-linked through corner-sharing of UO7 pentagonal bipyramids and PO3C tetrahedra forming a layer. The adjacent U···U distances within the layer are 0.5414 and 0.5743 nm. The organic groups of the 2-pmb3− ligand reside on the two sides of the layer. The interlayer space is filled with the racemic protonated phenethylamine cations to charge-balance the anionic layer. The shortest U···U distance between the layers is 1.239 nm. Compound 1 exhibits high thermal and water stability, especially in aqueous solution at pH 5~11 and in boiling water. The UV-Vis spectrum of 1 shows two broad bands peaking at 320 and 412 nm. The scalar-relativistic density functional calculations reveal that the two bands are associated with ligand-to-metal charge transfer (LMCT) transitions from ligand orbitals to metal orbitals lower-fδ (fz(x2−y2)). Photoluminescence properties show that 1 emits green-light at room temperature with six emission peaks at 481, 500, 516, 540, 564 and 591 nm, assigned to the electronic and vibronic transitions of S11-S00 and S10-S0ν (ν=0~4). Interestingly, both the emission intensity and the lifetime of compound 1 are temperature-dependent, making it possible to be used as a dual-response luminescence thermometer in the temperature range of 200~360 K. The intensity-dependent maximum sensitivity is 2.96%•K−1 (330 K) and the lifetime-dependent maximum sensitivity is 2.51%•K−1 (350 K), which are comparable to some lanthanide-based luminescent thermometers. This work provides a rare example of uranyl coordination polymers that can be used as a dual-response luminescence thermometer with wide operating temperature and good sensitivity.
Ge-Hua Wen , Wendurina , Xiumei Chen , Xiu-Fang Ma , Guo-Guo Weng , Yi-Fan Wei , Song-Song Bao , Xiaoji Xie , Shu-Xian Hu , Li-Min Zheng . Layered Uranyl Phosphonate as A Dual-response Luminescence Thermometer★[J]. Acta Chimica Sinica, 2023 , 81(10) : 1311 -1317 . DOI: 10.6023/A23050242
| [1] | Drami?anin, M. D. J. Appl. Phys. 2020, 128, 040902. |
| [2] | Yin, H.-Q.; Yin, X.-B. Acc. Chem. Res. 2020, 53, 485. |
| [3] | Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Chem. Rev. 2012, 112, 1126. |
| [4] | Heine, J.; Müller-Buschbaum, K. Chem. Soc. Rev. 2013, 42, 9232. |
| [5] | Brites, D.; Millán, A.; Carlos, L. D. In Lanthanides in Luminescent Thermometry, Vol. 49, Eds.: Bünzli, J.-C. G.; Pecharsky, V. K., Elsevier Science B.V., Amsterdam, 2016, p. 395. |
| [6] | Rocha, J.; Brites, C. D. S.; Carlos, L. D. Chem. Eur. J. 2016, 22, 14782. |
| [7] | Chambers, M. D.; Clarke, D. R. Annu. Rev. Mater. Res. 2009, 39, 325. |
| [8] | Hasegawa, Y.; Kitagawa, Y. J. Mater. Chem. C 2019, 7, 7494. |
| [9] | Kanzariya, D. B.; Chaudhary, M. Y.; Pal, T. K. Dalton Trans. 2023, 52, 7383. |
| [10] | Gálico, D. A.; Mazali, I. O.; Sigoli, F. A. J. Lumin. 2017, 192, 224. |
| [11] | Lapaev, D. V.; Nikiforov, V. G.; Lobkov, V. S.; Knyazev, A. A.; Galyametdinov, Y. G. Opt. Mater. 2018, 75, 787. |
| [12] | Cabral, F. M.; Gálico, D. A.; Mazali, I. O.; Sigoli, F. A. Inorg. Chem. Commun. 2018, 98, 29. |
| [13] | Zhao, D.; Yue, D.; Zhang, L.; Jiang, K.; Qian, G. Inorg. Chem. 2018, 57, 12596. |
| [14] | Chamberlain, T. W.; Perrella, R. V.; Oliveira, T. M.; de Sousa Filho, P. C.; Walton, R. I. Chem. Eur. J. 2022, 28, e202200410. |
| [15] | Wyczesany, M.; Zakrzewski, J. J.; Sieklucka, B.; Chorazy, S. J. Mater. Chem. C 2022, 10, 12054. |
| [16] | Mei, L.; An, S.-W.; Hu, K.-Q.; Wang, L.; Yu, J.-P.; Huang, Z.-W.; Kong, X.-H.; Xia, C.-Q.; Chai, Z.-F.; Shi, W.-Q. Angew. Chem. Int. Ed. 2020, 59, 16061. |
| [17] | Thuéry, P.; Atoini, Y.; Harroefield, J. Inorg. Chem. 2020, 59, 2503. |
| [18] | Gomez, G. E.; Ridenour, J. A.; Byrne, N. M.; Shevchenko, A. P.; Cahill, C. L. Inorg. Chem. 2019, 58, 7243. |
| [19] | Wu, S.; Mei, L.; Li, F.-Z.; An, S.-W.; Hu, K.-Q.; Nie, C.-M.; Chai, Z.-F.; Shi, W.-Q. Inorg. Chem. 2018, 57, 14772. |
| [20] | Yang, J.-J.; Zhao, Z.; Su, J. Inorg. Chem. 2023, 62, 1978. |
| [21] | Tang, S.-F.; Hou, X. Inorg. Chem. 2019, 58, 1382. |
| [22] | Zhao, R.; Li, F.-Z.; Yu, J.-P.; Mei, L.; Hu, K.-Q.; Chai, Z.-F.; Shi, W.-Q. Cryst. Growth Des. 2020, 20, 6966. |
| [23] | Zheng, T.; Gao, Y.; Chen, L.; Liu, Z.; Diwu, J.; Chai, Z.; Albrecht-Schmittc, T. E.; Wang, S. Dalton Trans. 2015, 44, 18158. |
| [24] | Zheng, T.; Gao, Y.; Chen, L.; Liu, Z.; Diwu, J.; Chai, Z.; Albrecht-Schmittc, T. E.; Wang, S. Inorg. Chim. Acta 2015, 435, 131. |
| [25] | Zheng, T.; Gao, Y.; Gui, D.; Chen, L.; Sheng, D.; Diwu, J.; Chai, Z.; Albrecht-Schmittc, T. E.; Wang, S. Dalton Trans. 2016, 45, 9031. |
| [26] | Wang, Y.; Wang, X.; Zhang, D.; Zhou, F.; Gui, D.; Zheng, T.; Li, J.; Chai, Z.; Wang, S. CrystEngComm. 2018, 20, 3153. |
| [27] | Wang, Y.; Zeng, D.; Zhou, F.; Zhang, D.; Li, J.; Zheng, T. J. Molecular Structure 2018, 1173, 183. |
| [28] | Zhao, H.; Qi, C.; Yan, X.; Ji, J.; Chai, Z.; Wang, S.; Zheng, T. ACS Appl. Mater. Interfaces 2022, 14, 14380. |
| [29] | Chen, L.; Zhang, Y.; Weng, Z.; Liu, Z.; Zhang, J.; Wang, Y.; Wang, S. Chin. J. Chem. 2021, 39, 597. |
| [30] | Gu, D.; Yang, W.; Chen, H.; Yang, Y.; Qin, X.; Chen, L.; Wang, S.; Pan, Q. Inorg. Chem. Front. 2021, 8, 3514. |
| [31] | Wen, G.-H.; Chen, X.-M.; Xu, K.; Xie, X.; Bao, S.-S.; Zheng, L.-M. Dalton Trans. 2021, 50, 17129. |
| [32] | Wen, G.-H.; Zou, Q.; Xu, K.; Huang, X.-D.; Bao, S.-S.; Chen, X.-T.; Ouyang, Z.; Wang, Z.; Zheng, L.-M. Chem. Eur. J. 2022, 28, e202200721. |
| [33] | Wen, G.-H.; Zou, Q.; Huang, X.-D.; Zhang, K.; Bao, S.-S.; Zheng, L.-M. Polyhedron 2021, 205, 115327. |
| [34] | Zhang, K.; Wen, G.-H.; Yang, X.-J.; Lim, D.-W.; Bao, S.-S.; Donoshita, M.; Wu, L.-Q.; Kitagawa, H.; Zheng, L.-M. ACS Materials Lett. 2021, 3, 744. |
| [35] | Knope, K. E.; Cahill, C. L. Inorg. Chem. 2008, 47, 7660. |
| [36] | Chen, L.; Chen, L.; Zhang, Y.; Xie, J.; Diwu, J. J. Inorg. Mater. 2020, 35, 1391 (in Chinese). |
| [36] | (陈磊, 陈兰花, 张瑜港, 谢健, 第五娟, 无机材料学报, 2020, 35, 1391.) |
| [37] | Liu, D.-D.; Wang, Y.-L.; Luo, F.; Liu, Q.-Y. Inorg. Chem. 2020, 59, 2952. |
| [38] | Wang, H.-Y.; Zheng, X.-Y.; Long, L.-S.; Kong, X.-J.; Zheng, L.-S. Inorg. Chem. 2021, 60, 6790. |
| [39] | Brites, C. D. S.; Lima, P. P.; Silva, N. J. O.; Millán, A.; Amaral, V. S.; Palacio, F.; Carlos, L. D. Nanoscale 2012, 4, 4799. |
| [40] | Ren, M.; Brites, C. D. S.; Bao, S.-S.; Ferreira, R. A. S.; Zheng, L.-M.; Carlos, L. D. J. Mater. Chem. C 2015, 3, 8480. |
| [41] | Guo, L.-R.; Tong, J.-W.; Liang, X.; K?hler, J.; Nuss, J.; Li, Y.-Z.; Zheng, L.-M. Dalton Trans. 2011, 40, 6392. |
| [42] | Goodwin, A. L.; Calleja, M.; Conterio, M. J.; Dove, M. T.; Evans, J. S. O.; Keen, D. A.; Peters, L.; Tucker, M. G. Science 2008, 319, 794. |
| [43] | Cliffe, M. J.; Goodwin, A. L. J. Appl. Cryst. 2012, 45, 1321. |
| [44] | Yang, X.-J.; Ren, M.; Bao, S.-S.; Hoshino, N.; Akutagawa, T.; Zheng, L.-M. Chem. Commun. 2014, 50, 3979. |
/
| 〈 |
|
〉 |