

Ir-catalyzed Vinylogous Aldol-type Condensation Reactions between Tertiary Amides and Siloxyfuran: the Synthesis of γ-Benzylidenebutenolides★
Received date: 2023-05-15
Online published: 2023-07-21
Supported by
National Natural Science Foundation of China(21931010)
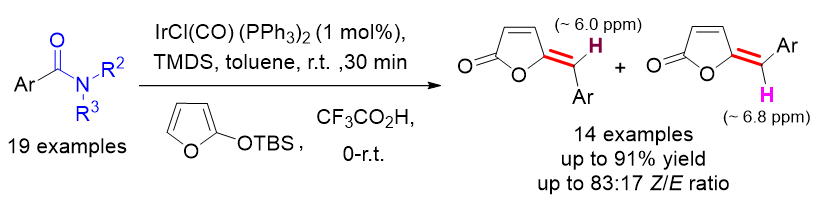
γ-Alkylidenebutenolides [5-alkylidene-2(5H)-furanones] are present in many biologically important natural products. One of the most direct and effective approaches to construct such compounds is the strategy based on vinylogous aldol condensation. However, this typically involves 2~3 reaction steps or a one-pot reaction carried out at high temperature. Compared to imines/iminiums and aldehydes, inert feedstock amides are more stable and readily available starting materials. Benefiting from the significant development of direct transformations of amides over the past few decades, amides have been well served as the surrogate of imine/iminium and aldehyde. In this work, we report a facile and efficient synthesis of γ-benzylidenebutenolides under mild conditions through Ir-catalyzed vinylogous aldol-type condensation between N,N-dimethyl arylformamides and silyloxyfuran. Nineteen examples of amides were transformed and fourteen examples of γ-benzylidenebutenolides were obtained in up to 91% yields and up to 83∶17 Z/E ratio. Notably, this reaction is well compatible with benzamides bearing electron-withdrawing substituents such as halogen, nitro, cyano, vinyl, and CF3 groups, with yields from 67% to 91% and Z/E ratios from 55∶45 to 83∶17. The general procedure is described as following: to a dried 10-mL round-bottom flask containing IrCl(CO)(PPh3)2 (4 mg, 0.50 mmol%) was added a tertiary amide (0.50 mmol) in toluene (2.5 mL) and 1,1,3,3-tetramethyldisiloxane (TMDS) (115 µL, 0.65 mmol) at room temperature. After being stirred for 30 min, the resulting mixture was cooled to 0 ℃. Then CF3CO2H (57 µL, 0.75 mmol) and tert-butyldimethylsilyloxyfuran (TBSOF) (248 mg, 1.25 mmol) were added dropwise successively. The mixture was allowed warm to room temperature, reacted for ca. 10 h. Upon completion of the reaction, the resulted mixture was concentrated under reduced pressure. The residue was purified by flash chromatography on silica gel to provide the corresponding γ-benzylidenebutenolide. Additionally, a summary of the chemical shift values for the characteristic 1H NMR peaks of γ-benzylidenebutenolides was provided, aiding in the verification of the isomers’ Z/E configuration. In the case of E-isomers, the benzylic hydrogen and α-hydrogen of the carbonyl produce 1H NMR peaks at around δ 6.68~6.82 and 6.30~6.50, respectively. Conversely, for Z-isomers, the respective resonances shift upfield by approximately δ 0.75 and 0.15. The transformation of γ-benzylidenebutenolides into γ-benzylbutyrolactones [5-benzyl-2(3H)-dihydrofuranone], which are core skeletons found in many bioactive compounds, can be expediently accomplished through Pd/C hydrogenation reaction.
Qian He , Jie Li , Sijia Yu , Dongping Wu , Jianliang Ye , Peiqiang Huang . Ir-catalyzed Vinylogous Aldol-type Condensation Reactions between Tertiary Amides and Siloxyfuran: the Synthesis of γ-Benzylidenebutenolides★[J]. Acta Chimica Sinica, 2023 , 81(10) : 1265 -1270 . DOI: 10.6023/A23050226
| [1] | (a) Pace, V.; Holzer, W. Aust. J. Chem. 2013, 66, 507. |
| [1] | (b) Pace, V.; Holzer, W.; Olofsson, B. Adv. Synth. Catal. 2014, 356, 3697. |
| [1] | (c) Volkov, A.; Tinnis, F.; Slagbrand, T.; Trillo, P.; Adolfsson, H. Chem. Soc. Rev. 2016, 45, 6685. |
| [1] | (d) Sato, T.; Yoritate, M.; Tajima, H.; Chida, N. Org. Biomol. Chem. 2018, 16, 3864. |
| [1] | (e) Kaiser, D.; Bauer, A.; Lemmerer, M.; Maulide, N. Chem. Soc. Rev. 2018, 47, 7899. |
| [1] | (f) Matheau-Raven, D.; Gabriel, P.; Leitch, J. A.; Almehmadi, Y. A.; Yamazaki, K.; Dixon, D. J. ACS Catal. 2020, 8880. |
| [2] | (a) Xiao, K.-J.; Luo, J.-M.; Ye, K.-Y.; Wang, Y.; Huang, P.-Q. Angew. Chem., Int. Ed. 2010, 49, 3037. |
| [2] | (b) Gregory, A. W.; Chambers, A.; Hawkins, A.; Jakubec, P.; Dixon, D. J. Chem. - Eur. J. 2015, 21, 111. |
| [2] | (c) Huang, P.-Q.; Ou, W.; Han, F. Chem. Commun. 2016, 52, 11967. |
| [2] | (d) Tinnis, F.; Volkov, A.; Slagbrand, T.; Adolfsson, H. Angew. Chem., Int. Ed. 2016, 55, 4562. |
| [2] | (e) Xie, L.-G.; Dixon, D. J. Chem. Sci. 2017, 8, 7492. |
| [2] | (f) Takahashi, Y.; Sato, T.; Chida, N. Chem. Lett. 2019, 48, 1138. |
| [2] | (g) Ou, W.; Huang, P.-Q. Sci. China: Chem. 2020, 63, 11. |
| [2] | (h) Yamazaki, K.; Gabriel, P.; Di Carmine, G.; Pedroni, J.; Farizyan, M.; Hamlin, T. A.; Dixon, D. J. ACS Catal. 2021, 11, 7489. |
| [2] | (i) Jiao, J.-W.; Wang, X.-M. Angew. Chem., Int. Ed. 2021, 60, 17088. |
| [2] | (j) Biallas, P.; Yamazaki, K.; Dixon, D. J. Org. Lett. 2022, 24, 2002. |
| [2] | (k) Wu, D.-P.; Ou, W.; Huang, P.-Q. Org. Lett. 2022, 24, 5366. |
| [2] | (l) Zhao, F.; Jiang, F.; Wang, X.-M. Sci. China: Chem. 2022, 65, 2231. |
| [2] | (m) He, Y.-L.; Wang, Y.-X.; Li, S.-J.; Lan, Y.; Wang, X.-M. Angew. Chem., Int. Ed. 2022, 61, e202115497. |
| [2] | (n) Yang, W.-H.; Jiao, J.-W.; Wang, X.-M. Chin. J. Org. Chem. 2023, 43, 1857 (in Chinese). |
| [2] | (杨雯涵, 焦继文, 王晓明, 有机化学, 2023, 43, 1857.) |
| [3] | (a) Soda, Y.; Sugiyama, Y.; Sato, S.; Shibuya, K.; Saegusa, J.; Matagawa, T.; Kawano, S.; Yoritate, M.; Fukaya, K.; Urabe, D.; Oishi, T.; Mori, K.; Simizu, S.; Chida, N.; Sato, T. Synthesis 2023, 55, 617. |
| [3] | (b) Soda, Y.; Sugiyama, Y.; Yoritate, M.; Tajima, H.; Shibuya, K.; Ogihara, C.; Oishi, T.; Sato, T.; Chida, N. Org. Lett. 2020, 22, 7502. |
| [3] | (c) Huang, X.-Z.; Gao, L.-H.; Huang, P.-Q. Nat. Commun. 2020, 11, 5314. |
| [3] | (d) Yoritate, M.; Takahashi, Y.; Tajima, H.; Ogihara, C.; Yokoyama, T.; Soda, Y.; Oishi, T.; Sato, T.; Chida, N. J. Am. Chem. Soc. 2017, 139, 18386. |
| [3] | (e) Guo, L.-D.; Hou, J.; Tu, W.; Zhang, Y.; Zhang, Y.; Chen, L.; Xu, J. J. Am. Chem. Soc. 2019, 141, 11713. |
| [3] | (f) Guo, L.-D.; Chen, Y.; Xu, J. Acc. Chem. Res. 2020, 53, 2726. |
| [3] | (g) Huang, Y.-H.; Liu, Z.-J.; Huang, P.-Q. Org. Chem. Front. 2022, 9, 58. |
| [3] | (h) Hu, J.-P.; Chen, W.-Q.; Jiang, Y.-Y.; Xu, J. Chin. J. Org. Chem. 2023, 43, 171 (in Chinese). |
| [3] | (胡晶平, 陈文清, 蒋宇旸, 徐晶, 有机化学, 2023, 43, 171.) |
| [4] | (a) Ruan, S.-T.; Luo, J.-M.; Du, Y.; Huang, P.-Q. Org. Lett. 2011, 13, 4938. |
| [4] | (b) Ye, J.-L.; Zhang, Y.-F.; Liu, Y.; Zhang, J.-Y.; Ruan, Y.-P.; Huang, P.-Q. Org. Chem. Front. 2015, 2, 697. |
| [5] | (a) Chen, H.; Wu, Z.-Z.; Shao, D.-Y.; Huang, P.-Q. Sci. Adv. 2022, 8, eade3431. |
| [5] | (b) Ou, W.; Han, F.; Hu, X.-N.; Chen, H.; Huang, P.-Q. Angew. Chem., Int. Ed. 2018, 57, 11354. |
| [5] | (c) Ji, K.-L.; He, S.-F.; Xu, D.-D.; He, W.-X.; Zheng, J.-F.; Huang, P.-Q. Angew. Chem., Int. Ed., 2023, 63, e202302832. |
| [6] | Nakajima, M.; Sato, T.; Chida, N. Org. Lett. 2015, 17, 1696. |
| [7] | (a) Chatterjee, S.; Sahoo, R.; Nanda, S. Org. Biomol. Chem. 2021, 19, 7298. |
| [7] | (b) Zhu, T.; Chen, Z.; Liu, P.; Wang, Y.; Xin, Z.; Zhu, W. J. Antibiot. 2014, 67, 315. |
| [7] | (c) Smitha, D.; Kumar, M. M. K.; Ramana, H.; Rao, D. V. Nat. Prod. Res. 2014, 28, 12. |
| [7] | (d) Sikorska, J.; Parker-Nance, S.; Davies-Coleman, M. T.; Vining, O. B.; Sikora, A. E.; McPhail, K. L. J. Nat. Prod. 2012, 75, 1824. |
| [7] | (e) Wang, W.; Kim, H.; Nam, S.-J.; Rho, B. J.; Kang, H. J. Nat. Prod. 2012, 75, 2049. |
| [7] | (f) Teixeira, R. R.; Barbosa, L. C. A.; Maltha, C. R. A.; Rocha, M. E.; Bezerra, D. P.; Costa-Lotufo, L. V.; Pessoa, C.; Moraes, M. O. Molecules 2007, 12, 1101. |
| [7] | (g) Manzanaro, S.; Salvá, J.; de la Fuente, J. á. J. Nat. Prod. 2006, 69, 1485. |
| [7] | (h) Fang, X. P.; Anderson, J. E.; Chang, C. J.; McLaughlin, J. L. Tetrahedron 1991, 47, 9751. |
| [7] | (i) Miao, S.; Andersen, R. J. J. Org. Chem. 1991, 56, 6275. |
| [7] | (j) Steffan, B.; Steglich, W. Angew. Chem., Int. Ed. 1984, 23, 445. |
| [8] | Aumann, D. C.; Clooth, G.; Steffan, B.; Steglich, W. Angew. Chem., Int. Ed. 1989, 28, 453. |
| [9] | (a) Guo, Z.; Bao, R.; Li, Y.; Li, Y.; Zhang, J.; Tang, Y. Angew. Chem., Int. Ed. 2021, 60, 14545. |
| [9] | (b) Yang, M.; Yin, F.; Fujino, H.; Snyder, S. A. J. Am. Chem. Soc. 2019, 141, 4515. |
| [10] | (a) Roy, D.; Tharra, P.; Baire, B. Org. Lett. 2021, 23, 5605. |
| [10] | (b) Li, B.-Z.; Chen, W.-M. Chin. J. Org. Chem. 2008, 28, 29 (in Chinese). |
| [10] | (李冰洲, 陈卫民, 有机化学, 2008, 28, 29.) |
| [10] | (c) Wang, B.-W.; Liu, Y.; Hao, Z.-F.; Hou, J.-Q.; Li, J.-Y.; Li, S.-T.; Pan, S.-H.; Zeng, M.-H.; Wang, Z.-Y. Chin. J. Org. Chem. 2018, 38, 1872 (in Chinese). |
| [10] | (王柏文, 刘园, 郝志峰, 侯佳琦, 李健怡, 李舒婷, 潘思慧, 曾铭豪, 汪朝阳, 有机化学, 2018, 38, 1872.) |
| [11] | Coperet, C.; Sugihara, T.; Wu, G.; Shimoyama, I.; Negishi, E.-I. J. Am. Chem. Soc. 1995, 117, 3422. |
| [12] | Inack-Ngi, S.; Rahmani, R.; Commeiras, L.; Chouraqui, G.; Thibonnet, J.; Duchêne, A.; Abarbri, M.; Parrain, J.-L. Adv. Synth. Catal. 2009, 351, 779. |
| [13] | (a) Chatterjee, S.; Acharyya, R. K.; Pal, P.; Nanda, S. Org. Biomol. Chem. 2022, 20, 2473. |
| [13] | (b) Rambabu, D.; Bhavani, S.; Nalivela, K. S.; Mukherjee, S.; Rao, M. V. B.; Pal, M. Tetrahedron Lett. 2013, 54, 2151. |
| [13] | (c) Lu, X.; Huang, X.; Ma, S. Tetrahedron Lett. 1993, 34, 5963. |
| [14] | Yu, C.; Zhang, J.; Zhong, G. Chem. Commun. 2017, 53, 9902. |
| [15] | (a) Casiraghi, G.; Zanardi, F.; Appendino, G.; Rassu, G. Chem. Rev. 2000, 100, 1929. |
| [15] | (b) Khotavivattana, T.; Khamkhenshorngphanuch, T.; Rassamee, K.; Siripong, P.; Vilaivan, T. Tetrahedron Lett. 2018, 59, 2711. |
| [15] | (c) Kuse, M.; Moriguchi, M.; Hachida, M.; Takikawa, H. Chem. Lett. 2017, 46, 1409. |
| [15] | (d) Chaubet, G.; Goh, S. S.; Mohammad, M.; Gockel, B.; Cordonnier, M.-C. A.; Baars, H.; Phillips, A. W.; Anderson, E. A. Chem. - Eur. J. 2017, 23, 14080. |
| [15] | (e) Boukouvalas, J.; Beltran, P. P.; Lachance, N.; Cote, S.; Maltais, F.; Pouliot, M. Synlett 2007, 219. |
| [15] | (f) Kong, K.; Romo, D. Org. Lett. 2006, 8, 2909. |
| [15] | (g) Vaz, B.; Alvarez, R.; Brückner, R.; de Lera, A. R. Org. Lett. 2005, 7, 545. |
| [15] | (h) Fiorenza, M.; Reginato, G.; Ricci, A.; Taddei, M.; Dembech, P. J. Org. Chem. 1984, 49, 551. |
| [15] | (i) Asaoka, M.; Yanagida, N.; Ishibashi, K.; Takei, H. Tetrahedron Lett. 1981, 22, 4269. |
| [16] | (a) Schacht, M.; Boehlich, G. J.; de Vries, J.; Bertram, S.; Gabriel, G.; Zimmermann, P.; Heisig, P.; Schuetzenmeister, N. Eur. J. Org. Chem. 2017, 1745. |
| [16] | (b) Teixeira, R. R.; Barbosa, L. C. A.; Forlani, G.; Pilo-Veloso, D.; Carneiro, J. W. M. J. Agric. Food Chem. 2008, 56, 2321. |
| [16] | (c) Teixeira, R. R.; Barbosa, L. C. A.; Santana, J. O.; Veloso, D. P.; Ellena, J.; Doriguetto, A. C.; Drew, M. G. B.; Ismail, F. M. D. J. Mol. Struct. 2007, 837, 197. |
| [17] | Kotora, M.; Negishi, E. I. Synthesis 1997, 121. |
| [18] | Lambert, J. D.; Rice, J. E.; Hong, J.; Hou, Z.; Yang, C. S. Bioorg. Med. Chem. Lett. 2005, 15, 873. |
| [19] | (a) Gogoi, S.; Argade, N. P. Tetrahedron 2006, 62, 2715. |
| [19] | (b) Wu, L.; Zhang, Z.; Liao, J.; Li, J.; Wu, W.; Jiang, H. Chem. Commun. 2016, 52, 2628. |
| [20] | (a) Li, M.-L.; Li, Y.; Pan, J.-B.; Li, Y.-H.; Song, S.; Zhu, S.-F.; Zhou, Q.-L. ACS Catal. 2020, 10, 10032. |
| [20] | (b) Shu, C.; Liu, M.-Q.; Sun, Y.-Z.; Ye, L.-W. Org. Lett. 2012, 14, 4958. |
/
| 〈 |
|
〉 |