

Tyrosine Derivative Regulated Enzyme Catalytic Pathway for Controllable Synthesis of Functional Melanin★
Received date: 2023-05-19
Online published: 2023-07-28
Supported by
National Natural Science Foundation of China(22025207); National Natural Science Foundation of China(22232006); National Natural Science Foundation of China(22077122); National Natural Science Foundation of China(22072004)
Construction of biomacromolecules via enzyme-mediated catalytic assembly from small biomolecules is fascinating for preparing functional biological materials. The challenge remains on how to control the structure and functions of biomacromolecules through substrate regulation. A sequence of crucial biomacromolecules, melanin, were prepared via simple substrate derivation, which controls the key polymerization sites in enzyme catalyzed self-assembly process. In detail, we designed three tyrosine derivatives, namely, 3-fluorotyrosine [Tyr(F)], N-acetyltyrosine [Tyr(N-Ac)], and tyrosine ethyl ester [Tyr(OEt)]. The three substrates corresponded to the blockage of different tyrosinase-mediated polymerization active site, and tyrosine was used as the reference. The above small molecules as substrates (1.0 mmol/L) and tyrosinase (2 U) were mixed in phosphate buffer (pH=8.5, 0.10 mol/L, 2.0 mL), which was stirred in an air environment of 25 ℃. After 24 h, the reaction was quenched and the mixture was centrifuged to obtain different melanin nanoparticle products (MNPs). Characterizations from transmission electron microscopy (TEM), infrared spectroscopy (IR), and X-ray photoelectron spectroscopy (XPS) showed that all the melanin products had eumelanin-like skeleton, but were different in the degree of polymerization and microscopic chemical structures. These structure-modified MNPs showed overall absorption in the ultraviolet-visible-near infrared (UV-vis-NIR) region, thus enabling photothermal conversion in the NIR-I region. The photothermal conversion efficiency of MNP, MNP(F), and MNP(OEt) (3 mg•mL-1) was measured to be 46.6%, 37.0%, and 25.8% [laser 808 nm, 1.5 W, where MNP(N-Ac) was not available due to rather low temperature increase]. Interestingly, in vitro experiment showed that MNP(OEt) exhibited better photothermal cytotoxicity than MNP, and this was probably because MNP(OEt) had a smaller particle size and less negative ζ-potential, which could ease cell endocytosis. This work demonstrated the feasibility to regu-late enzyme-mediated catalytic pathway via simple substrate derivation. It provides insights for the construction of new functional melanin materials and for revealing the relationship between biological macromolecular structure and function.
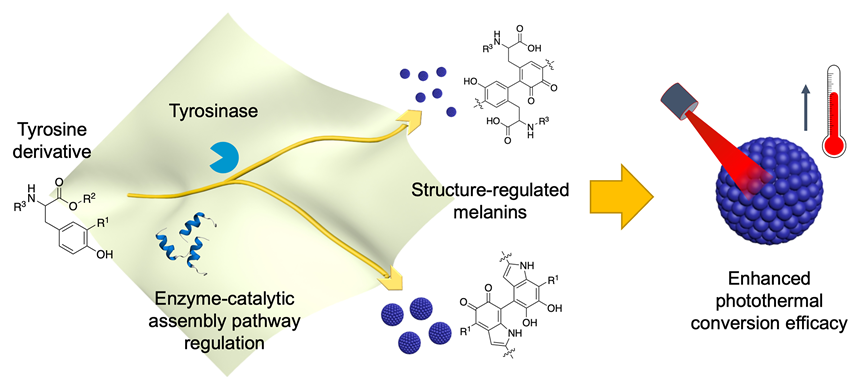
Dongrui Su , Xiaokang Ren , Yunhao Yu , Luyang Zhao , Tianyu Wang , Xuehai Yan . Tyrosine Derivative Regulated Enzyme Catalytic Pathway for Controllable Synthesis of Functional Melanin★[J]. Acta Chimica Sinica, 2023 , 81(11) : 1486 -1492 . DOI: 10.6023/A23050240
| [1] | Sorrenti A.; Leira-Iglesias J.; Markvoort A. J.; de Greef T. F. A.; Hermans T. M. Chem. Soc. Rev. 2017, 46, 5476. |
| [2] | Li Y.; Sun P.; Zhao L.; Yan X.; Ng D. K. P.; Lo P. C. Angew. Chem., Int. Ed. 2020, 59, 23228. |
| [3] | Chen K.; Xing R.; Yan X. Aggregate 2021, 2, 84. |
| [4] | Han J.; Liu K.; Chang R.; Zhao L.; Yan X. Angew. Chem., Int. Ed. 2019, 58, 2000. |
| [5] | Lampel A.; Mcphee S. A.; Park H.; Scott G. G.; Humagain S.; Hekstra D. R.; Yoo B.; Frederix P. W. J. M.; Li T.; Abzalimov R. R.; Greenbaum S. G.; Tuttle T.; Hu C.; Bettinger C. J.; Ulijn R. V. Science 2017, 1068, 1064. |
| [6] | Wang Y.; Dong W. Mater. China 2019, 38, 470. (in Chinese) |
| [6] | 汪洋, 东为富, 中国材料进展, 2019, 38, 470). |
| [7] | Ren Y.; Yang L.; Gao L.; Wang F.; Shi N.; Zhao Y.; Guo L.; Wang H. Mater. Rep. 2020, 34, 11145. (in Chinese) |
| [7] | 任燕玲, 杨柳, 高莉, 王芳, 史楠, 赵英虎, 郭丽晓, 王海宾, 材料导报, 2020, 34, 11145). |
| [8] | Zhou J.; Wang H.; Tong L. Chin. Sci. Bull. 2023, 68, 1406 (in Chinses). |
| [8] | 周建良, 王怀雨, 童丽萍, 科学通报, 2023, 68, 1406). |
| [9] | Yang P.; Zhang S.; Zhang N.; Wang Y.; Zhong J.; Sun X.; Qi Y.; Chen X.; Li Z.; Li Y. ACS Appl. Mater. Interfaces 2019, 11, 42671. |
| [10] | D’Ischia M.; Napolitano A.; Ball V.; Chen C. T.; Buehler M. J. Acc. Chem. Res. 2014, 47, 3541. |
| [11] | Cao W.; Zhou X.; McCallum N. C.; Hu Z.; Ni Q. Z.; Kapoor U.; Heil C. M.; Cay K. S.; Zand T.; Mantanona A. J.; Jayaraman A.; Dhinojwala A.; Deheyn D. D.; Shawkey M. D.; Burkart M. D.; Rinehart J. D.; Gianneschi N. C. J. Am. Chem. Soc. 2021, 143, 2622. |
| [12] | Partlow B. P.; Applegate M. B.; Omenetto F. G.; Kaplan D. L. ACS Biomater. Sci. Eng. 2016, 2, 2108. |
| [13] | McCallum N. C.; Son F. A.; Clemons T. D.; Weigand S. J.; Gnanasekaran K.; Battistella C.; Barnes B. E.; Abeyratne-Perera H.; Siwicka Z. E.; Forman C. J.; Zhou X.; Moore M. H.; Savin D. A.; Stupp S. I.; Wang Z.; Vora G. J.; Johnson B. J.; Farha O. K.; Gianneschi N. C. J. Am. Chem. Soc. 2021, 143, 4005. |
| [14] | Cao W.; McCallum N. C.; Ni Q. Z.; Li W.; Boyce H.; Mao H.; Zhou X.; Sun H.; Thompson M. P.; Battistella C.; Wasielewski M. R.; Dhinojwala A.; Shawkey M. D.; Burkart M. D.; Wang Z.; Gianneschi N. C. J. Am. Chem. Soc. 2020, 142, 12802. |
| [15] | Della Vecchia N. F.; Luchini A.; Napolitano A.; D’Errico G.; Vitiello G.; Szekely N.; d’Ischia M.; Paduano L. Langmuir 2014, 30, 9811. |
| [16] | Ren X.; Zhao L.; Yuan C.; Shi M.; Xing R. Chem. Eng. J. 2022, 450, 138293. |
| [17] | Ren X.; Zou Q.; Yuan C.; Chang R.; Xing R.; Yan X. Angew. Chem., Int. Ed. 2019, 58, 5872. |
| [18] | Ito S. Pigment Cell Res. 2003, 16, 230. |
| [19] | Ito S.; Wakamatsu K. Photochem. Photobiol. 2008, 84, 582. |
| [20] | Lampel A.; McPhee S. A.; Kassem S.; Sementa D.; Massarano T.; Aramini J. M.; He Y.; Ulijn R. V. Angew. Chem., Int. Ed. 2021, 60, 7564. |
| [21] | Zhao L.; Ren X.; Yan X. CCS Chem. 2021, 3, 678. |
| [22] | Yasunobu K. T.; Peterson E. W.; Mason H. S. J. Biol. Chem. 1959, 234, 3291. |
| [23] | Ramsden C. A.; Riley P. A. Bioorganic Med. Chem. 2014, 22, 2388. |
| [24] | Yang P.; Gu Z.; Zhu F; Li Y. CCS Chem. 2020, 2, 128. |
| [25] | Zong S.; Wang L.; Yang Z.; Wang H.; Wang Z.; Cui Y. ACS Appl. Mater. Interfaces 2019, 11, 5896. |
| [26] | Xi Z. Y.; Xu Y. Y.; Zhu L. P.; Wang Y.; Zhu B. K. J. Membr. Sci. 2009, 327, 244. |
| [27] | Panzella L.; Gentile G.; D’Errico G.; Della Vecchia N. F.; Errico M. E.; Napolitano A.; Carfagna C.; D’Ischia M. Angew. Chem., Int. Ed. 2013, 52, 12684. |
| [28] | Stypczyńska A.; Nixon T.; Mason N. Eur. Phys. J. D 2014, 68, 333. |
| [29] | Ding Y.; Weng L.-T.; Yang M.; Yang Z.; Lu X.; Huang N.; Leng Y. Langmuir 2014, 30, 12258. |
| [30] | Guo C.; Wang B.; Ma X. Acta Chim. Sinica 2021, 79, 967. (in Chinese) |
| [30] | 郭彩霞, 王博, 马小杰, 化学学报, 2021, 79, 967). |
| [31] | Zou Q.; Abbas M.; Zhao L.; Li S.; Shen G.; Yan X. J. Am. Chem. Soc. 2017, 139, 1921. |
| [32] | Zhao L.; Zou Q.; Yan X. Bull. Chem. Soc. Jpn. 2019, 92, 70. |
| [33] | Chang R.; Zhao L.; Xing R.; Li J.; Yan X. Chem. Soc. Rev. 2023, 52, 2688. |
| [34] | Chang R.; Zou Q.; Zhao L.; Liu Y.; Xing R.; Yan X. Adv. Mater. 2022, 34, 2200139. |
| [35] | Li S.; Zhang W.; Xing R.; Yuan C.; Xue H.; Yan X. Adv. Mater. 2021, 33, 2100595. |
| [36] | Zhao L.; Liu Y.; Chang R.; Xing R.; Yan X. Adv. Funct. Mater. 2019, 29, 1806877. |
| [37] | Zhao L.; Liu Y.; Xing R.; Yan X. Angew. Chem., Int. Ed. 2020, 59, 3793. |
| [38] | Qiu B.; Xue L.; Yang Y.; Bin H.; Zhang Y.; Zhang C.; Xiao M.; Park K.; Morrison W.; Zhang Z. G.; Li Y. Chem. Mater. 2017, 29, 7543. |
| [39] | Yang Y.; Zhang Z. G.; Bin H.; Chen S.; Gao L.; Xue L.; Yang C.; Li Y. J. Am. Chem. Soc. 2016, 138, 15011. |
/
| 〈 |
|
〉 |