

Study on Main Chain Structure Regulation and Properties of Conjugated Copolymers Based on 2,6-Azulene and 3,4-Propylenedioxythiophene★
Received date: 2023-06-16
Online published: 2023-08-18
Supported by
National Natural Science Foundation of China(22225506); National Natural Science Foundation of China(22075310); Youth Innovation Promotion Association CAS(2022252)
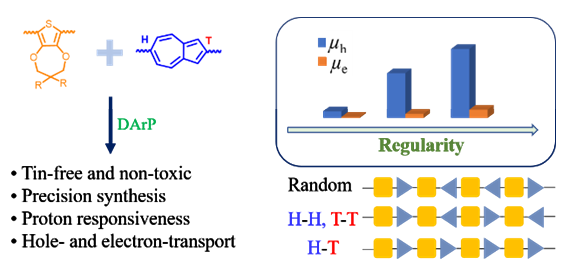
Azulene has attracted significant attention for constructing novel optoelectronic materials. Tuning the dipole orientation of azulene unit in azulene-based conjugated polymers has recently aroused widespread concern and remains a great challenge due to the lack of synthetic method. Herein, we report three 2,6-azulene and 3,4-propylenedioxythiophene (ProDOT) based conjugated copolymers P(AzProDOT-1), P(AzProDOT-2) and P(AzProDOT-3) with different dipole arrangements of azulene moieties. The regioregularity of these 2,6-azulene-ProDOT-based conjugated polymers was tuned by monomer design and direct arylation polymerization strategy, which enables a thorough study of the impact of the regioregularity on the properties of these polymers and their charge transport performance. The dipole orientation of 2,6-azulene units were regiorandom for P(AzProDOT-1), regularity with medium regioregularity for P(AzProDOT-2) and regularity with high regioregularity for P(AzProDOT-3), respectively. The number-average molecular weight values of P(AzProDOT-1), P(AzProDOT-2) and P(AzProDOT-3) estimated by gel permeation chromatography (GPC) were 11.1, 11.4 and 9.3 kDa, respectively, and the chemical structures of these three polymers were also characterized by high-temperature 1H NMR spectra. Ultraviolet-visible (UV-vis) absorption spectra and cyclic voltammetry were conducted to evaluate the optoelectronic properties of these polymers. The blue-shift of the maximum absorption peak for P(AzProDOT-2) indicates its twisted polymer backbone and short effective π-conjugation length, while the red-shift of the maximum absorption peak for P(AzProDOT-3) demonstrates the more planar conjugated skeleton and the longer effective π-conjugation length, although its molecular weight was a little lower. Besides, there was a prominent shoulder peak in the thin film of P(AzProDOT-3) in UV-vis absorption spectrum, indicating the stronger interchain interactions in solid state. All these observations were in agreement with the density functional theory (DFT) calculation results. Due to the electron-donating property of ProDOT, these three polymers displayed strong and sensitive proton responsiveness. The ultraviolet-visible-near infrared (UV-vis-NIR) spectra of these three polymers showed obvious red-shifts (>150 nm) upon protonation, and the films of these polymers also possess strong proton responsiveness properties. Charge-carrier mobilities of these three polymers were measured by the space-charge-limited current (SCLC). The hole mobilities of thin films of P(AzProDOT-1), P(AzProDOT-2) and P(AzProDOT-3) were 1.32×10−5, 9.14×10−5 and 1.41×10−4 cm2•V−1•s−1, respectively, and their electron mobilities were 1.62×10−6, 7.91×10−6 and 1.66×10−5 cm2•V−1•s−1, respectively. The atomic force microscopy (AFM) study demonstrated that the thin film of P(AzProDOT-3) possessed the smoothest surface and the smallest root mean square (RMS) roughness, proving the optimal SCLC performance of P(AzProDOT-3) among these three polymers. Our research highlights the significant and effective strategy of rational control regioregularity of azulene-based copolymer backbone to tune physicochemical properties and molecular packing for achieving better charge transport performance. Our study also aims to give valuable insight into precision synthesis of regioregular conjugated polymers based on low-symmetric conjugated building blocks.
Yang Wang , Junjun Xiang , Congwu Ge , Xike Gao . Study on Main Chain Structure Regulation and Properties of Conjugated Copolymers Based on 2,6-Azulene and 3,4-Propylenedioxythiophene★[J]. Acta Chimica Sinica, 2023 , 81(10) : 1341 -1349 . DOI: 10.6023/A23060292
| [1] | Lemal, D. M.; Goldman, G. D. J. Chem. Educ. 1988, 65, 923. |
| [2] | (a) Koch, M.; Blacque, O.; Venkatesan, K. Org. Lett. 2012, 14, 1580. |
| [2] | (b) Tang, T.; Lin, T.; Wang, F.; He, C. J. Phys. Chem. B 2015, 119, 8176. |
| [2] | (c) Wang, F.; Lin, T. T.; He, C.; Chi, H.; Tang, T.; Lai, Y.-H. J. Mater. Chem. 2012, 22, 10448. |
| [2] | (d) Wang, F.; Lai, Y. H.; Kocherginsky, N. M.; Kosteski, Y. Y. Org. Lett. 2003, 5, 995. |
| [3] | (a) Anderson, A. G.; Steckler, B. M. J. Am. Chem. Soc. 1959, 81, 4941. |
| [3] | (b) Michl, J.; Thulstrup, E. W. Tetrahedron 1976, 32, 205. |
| [4] | (a) Xin, H.; Li, J.; Lu, R. Q.; Gao, X.; Swager, T. M. J. Am. Chem. Soc. 2020, 142, 13598. |
| [4] | (b) Ran, H.; Duan, X.; Zheng, R.; Xie, F.; Chen, L.; Zhao, Z.; Han, R.; Lei, Z.; Hu, J. Y. ACS Appl. Mater. Interfaces 2020, 12, 23225. |
| [4] | (c) Yamaguchi, Y.; Maruya, Y.; Katagiri, H.; Nakayama, K.; Ohba, Y. Org. Lett. 2012, 14, 2316. |
| [4] | (d) Hou, B.; Li, J.; Xin, H.; Yang, X.; Gao, H.; Peng, P.; Gao, X. Acta Chim. Sinica 2020, 78, 788 (in Chinese). |
| [4] | (侯斌, 李晶, 辛涵申, 杨笑迪, 高洪磊, 彭培珍, 高希珂, 化学学报, 2020, 78, 788) |
| [4] | (e) Peng, P.; Li, J.; Hou, B.; Xin, H.; Cheng, T.; Gao, X. Chin. J. Org. Chem. 2020, 40, 3916 (in Chinese). |
| [4] | (彭培珍, 李晶, 侯斌, 辛涵申, 程探宇, 高希珂, 有机化学, 2020, 40, 3916) |
| [5] | (a) Yao, J.; Cai, Z.; Liu, Z.; Yu, C.; Luo, H.; Yang, Y.; Yang, S.; Zhang, G.; Zhang, D. Macromolecules 2015, 48, 2039. |
| [5] | (b) Xin, H.; Li, J.; Ge, C.; Yang, X.; Xue, T.; Gao, X. Mater. Chem. Front. 2018, 2, 975. |
| [5] | (c) Chen, Y.; Zhu, Y.; Yang, D.; Zhao, S.; Zhang, L.; Yang, L.; Wu, J.; Huang, Y.; Xu, Z.; Lu, Z. Chemistry 2016, 22, 14527. |
| [6] | (a) Tzoganakis, N.; Feng, B.; Loizos, M.; Krassas, M.; Tsikritzis, D.; Zhuang, X.; Kymakis, E. J. Mater. Chem. C 2021, 9, 14709. |
| [6] | (b) Su, Y.; Li, H.; Miao, Y.; Tian, Y.; Cheng, M. Asian. J. Org. Chem. 2022, 11, e202200441. |
| [6] | (c) Zhu, W.; Zhou, K.; Fo, Y.; Li, Y.; Guo, B.; Zhang, X.; Zhou, X. Phys. Chem. Chem. Phys. 2022, 24, 18793. |
| [7] | (a) Umeyama, T.; Watanabe, Y.; Miyata, T.; Imahori, H. Chem. Lett. 2015, 44, 47. |
| [7] | (b) Wang, X.; Ng, J. K.-P.; Jia, P.; Lin, T.; Cho, C. M.; Xu, J.; Lu, X.; He, C. Macromolecules 2009, 42, 5534. |
| [7] | (c) Wang, F.; Lai, Y.-H.; Han, M.-Y. Macromolecules 2004, 37, 3222. |
| [7] | (d) Wang, F.; Lai, Y.-H. Macromolecules 2003, 36, 536. |
| [8] | Murai, M.; Amir, E.; Amir, R. J.; Hawker, C. J. Chem. Sci. 2012, 3, 2721. |
| [9] | Xin, H.; Hou, B.; Gao, X. Acc. Chem. Res. 2021, 54, 1737. |
| [10] | (a) Yamaguchi, Y.; Ogawa, K.; Nakayama, K.; Ohba, Y.; Katagiri, H. J. Am. Chem. Soc. 2013, 135, 19095. |
| [10] | (b) Yamaguchi, Y.; Takubo, M.; Ogawa, K.; Nakayama, K.; Koganezawa, T.; Katagiri, H. J. Am. Chem. Soc. 2016, 138, 11335. |
| [11] | Xiang, J.; Tan, W. L.; Zhang, J.; Wang, Y.; Duan, C.; McNeill, C. R.; Yang, X.; Ge, C.; Gao, X. Macromolecules 2022, 55, 8074. |
| [12] | (a) Gao, H.; Ge, C.; Hou, B.; Xin, H.; Gao, X. ACS Macro Lett. 2019, 8, 1360. |
| [12] | (b) Hou, B.; Zhou, Z.; Yu, C.; Xue, X. S.; Zhang, J.; Yang, X.; Li, J.; Ge, C.; Wang, J.; Gao, X. ACS Macro Lett. 2022, 11, 680. |
| [13] | (a) Homyak, P.; Liu, Y.; Liu, F.; Russel, T. P.; Coughlin, E. B. Macromolecules 2015, 48, 6978. |
| [13] | (b) Gobalasingham, N. S.; Pankow, R. M.; Ekiz, S.; Thompson, B. C. J. Mater. Chem. A 2017, 5, 14101. |
| [13] | (c) Broll, S.; Nübling, F.; Luzio, A.; Lentzas, D.; Komber, H.; Caironi, M.; Sommer, M. Macromolecules 2015, 48, 7481. |
| [13] | (d) Akkuratov, A. V.; Prudnov, F. A.; Chernyak, A. V.; Kuznetsov, P. M.; Peregudov, A. S.; Troshin, P. A. J. Polym. Sci., Part A: Polym. Chem. 2019, 57, 776. |
| [14] | (a) DiTullio, B. T.; Savagian, L. R.; Bardagot, O.; De Keersmaecker, M.; Osterholm, A. M.; Banerji, N.; Reynolds, J. R. J. Am. Chem. Soc. 2023, 145, 122. |
| [14] | (b) Mi, S.; Wu, J.; Liu, J.; Xu, Z.; Wu, X.; Luo, G.; Zheng, J.; Xu, C. ACS Appl. Mater. Interfaces 2015, 7, 27511. |
| [14] | (c) Luo, X.; Shen, H.; Perera, K.; Tran, D. T.; Boudouris, B. W.; Mei, J. ACS Macro Lett. 2021, 10, 1061. |
| [14] | (d) Yang, W.; Yue, H.-G.; Zhao, D.; Yan, H.; Cao, K.-L.; Zhao, J.-S.; Zhang, Q. Chinese J. Polym. Sci. 2021, 39, 147. |
| [15] | Meager, I.; Ashraf, R. S.; Rossbauer, S.; Bronstein, H.; Donaghey, J. E.; Marshall, J.; Schroeder, B. C.; Heeney, M.; Anthopoulos, T. D.; McCulloch, I. Macromolecules 2013, 46, 5961. |
| [16] | Fan, Q.; Martin-Jimenez, D.; Ebeling, D.; Krug, C. K.; Brechmann, L.; Kohlmeyer, C.; Hilt, G.; Hieringer, W.; Schirmeisen, A.; Gottfried, J. M. J. Am. Chem. Soc. 2019, 141, 17713. |
| [17] | Hou, B.; Li, J.; Zhou, Z. F.; Tan, W. L.; Yang, X. D.; Zhang, J. W.; McNeill, C. R.; Ge, C. W.; Wang, J. T.; Gao, X. K. ACS Mater. Lett. 2022, 4, 392. |
| [18] | Wang, Y.; Tan, W. L.; Xiang, J.; Ge, C.; McNeill, C. R.; Gao, X. ACS Macro Lett. 2023, 12, 487. |
| [19] | (a) Dudnik, A. S.; Aldrich, T. J.; Eastham, N. D.; Chang, R. P.; Facchetti, A.; Marks, T. J. J. Am. Chem. Soc. 2016, 138, 15699. |
| [19] | (b) Wakioka, M.; Ishiki, S.; Ozawa, F. Macromolecules 2015, 48, 8382. |
| [19] | (c) Aldrich, T. J.; Dudnik, A. S.; Eastham, N. D.; Manley, E. F.; Chen, L. X.; Chang, R. P. H.; Melkonyan, F. S.; Facchetti, A.; Marks, T. J. Macromolecules 2018, 51, 9140. |
| [20] | (a) Zhang, C.; Matos, T.; Li, R.; Sun, S.-S.; Lewis, J. E.; Zhang, J.; Jiang, X. Polymer Chemistry 2010, 1, 663. |
| [20] | (b) Wang, M.; Wang, H.; Yokoyama, T.; Liu, X.; Huang, Y.; Zhang, Y.; Nguyen, T. Q.; Aramaki, S.; Bazan, G. C. J. Am. Chem. Soc. 2014, 136, 12576. |
| [21] | Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A. V.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery Jr, J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M. J.; Heyd, J. J.; Brothers, E. N.; Kudin, K. N.; Staroverov, V. N.; Keith, T. A.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A. P.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J. Gaussian 16, Wallingford CT, 2016. |
| [22] | Kim, J. S.; Fei, Z.; Wood, S.; James, D. T.; Sim, M.; Cho, K.; Heeney, M. J.; Kim, J.-S. Adv. Energy Mater. 2014, 4, 1400527. |
| [23] | Más-Montoya, M.; Janssen, R. A. J. Adv. Funct. Mater. 2017, 27, 1605779. |
| [24] | Kang, S. H.; Lee, D.; Kim, H.; Choi, W.; Oh, J.; Oh, J. H.; Yang, C. ACS Appl. Mater. Interfaces 2021, 13, 52840. |
| [25] | (a) Tu, Q.; Ma, Y.; Zhou, X.; Ma, W.; Zheng, Q. Chem. Mater. 2019, 31, 5953. |
| [25] | (b) Huang, H.; Bin, H.; Peng, Z.; Qiu, B.; Sun, C.; Liebman-Pelaez, A.; Zhang, Z.-G.; Zhu, C.; Ade, H.; Zhang, Z.; Li, Y. Macromolecules 2018, 51, 6028. |
| [26] | Brown, A. R.; Jarrett, C. P.; de Leeuw, D. M.; Matters, M. Synth. Met. 1997, 88, 37. |
| [27] | Tsurui, K.; Murai, M.; Ku, S.-Y.; Hawker, C. J.; Robb, M. J. Adv. Funct. Mater. 2014, 24, 7338. |
| [28] | Wang, Y.; Liu, Y.; Chen, S.; Peng, R.; Ge, Z. Chem. Mater. 2013, 25, 3196. |
/
| 〈 |
|
〉 |