

Recent Advances in Radical-Based Dehydroxylation of Hydroxyl Groups via Oxalates
Received date: 2023-07-15
Online published: 2023-08-24
Supported by
National Natural Science Foundation of China(22201201); Natural Science Foundation of Zhejiang Province(LY23B020001)
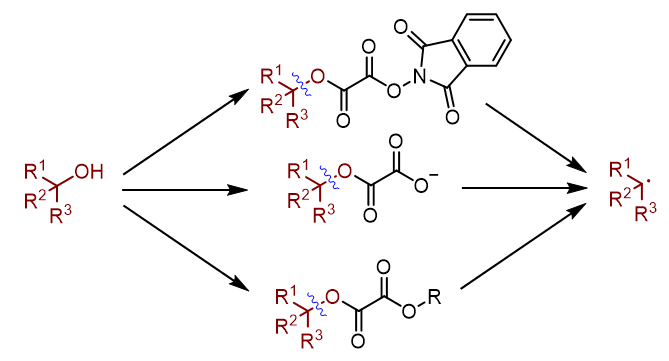
Alcohols are among the most synthetically versatile, operationally convenient, and commercially abundant functional groups in modern organic chemistry, which are widely present in a variety of drugs, natural products, agrochemicals, and fine chemicals. Alkyl radicals are the most important building blocks in radical chemistry. Traditionally, the alkyl radicals can be generated from the alcohols through the Barton-McCombie radical deoxygenation reaction. Despite the importance of this reaction, one of the several limitations of this strategy is the requirement of stoichiometric quantities of hazardous reagents. Another limitation is that the generated alkyl radical can abstract a hydrogen atom from tributyltin hydride to deliver an alkane. Therefore, development of a convenient and compatible method for generation of alkyl radicals from alcohols has synthetic appeal, yet it represents a long-term challenge and urgent demand. With the development of organic chemistry, much efforts have been devoted to the development of this transformation. This paper reviews the recent advances in dehydroxylation of hydroxyl groups via oxalates, focuses on the design principles and concepts of these strategies, compares the reaction mechanisms, and summarizes the generalities and differences of these methods. It is divided into three sections consisting of the radical-based dehydroxylation of hydroxyl groups via N‑phthalimidoyl oxalates, oxalate monoester salts and oxalates. The future and trend of radical-based dehydroxylation of hydroxyl groups in organic chemistry were prospected.
Key words: photocatalysis; free radicals; dehydroxylation; alcohols; oxalates
Jianqiang Chen , Gangguo Zhu , Jie Wu . Recent Advances in Radical-Based Dehydroxylation of Hydroxyl Groups via Oxalates[J]. Acta Chimica Sinica, 2023 , 81(11) : 1609 -1623 . DOI: 10.6023/A23070339
| [1] | (a) Ertl P.; Schuhmann T. J. Nat. Prod. 2019, 82, 1258. |
| [1] | (b) Henkel T.; Brunne R. M.; Müller H.; Reichel F. Angew. Chem. Int. Ed. 1999, 38, 643. |
| [2] | For reviews, see: (a) Hatwig W. Tetrahedron 1983, 39, 2609. |
| [2] | (b) Crich D.; Quintero L. Chem. Rev. 1989, 89, 1413. |
| [3] | (a) Zhang Z.; Gong L.; Zhou X.-Y.; Yan S.-S.; Li J.; Yu D.-G. Acta Chim. Sinica 2019, 77, 783.. (in Chinese) |
| [3] | ( 张振, 龚莉, 周晓渝, 颜思顺, 李静, 余达刚, 化学学报, 2019, 77, 783.) |
| [3] | (b) Chen J.-Q.; Tu X.; Tang Q.; Li K.; Xu L.; Wang S.; Ji M.; Li Z.; Wu J. Nat. Commun. 2021, 12, 5328. |
| [3] | (c) Ji M.; Xu L.; Luo X.; Jiang M.; Wang S.; Chen J.; Wu J. Org. Chem. Front. 2021, 8, 6704. |
| [3] | (d) Yang M.; Ye B.; Chen J.; Wu J. Acta Chim. Sinica 2022, 80, 11.. (in Chinese) |
| [3] | ( 杨民, 叶柏柏, 陈健强, 吴劼, 化学学报, 2022, 80, 11.) |
| [3] | (e) Chen J.-Q.; Tu X.; Qin B.; Huang S.; Zhang J.; Wu J. Org. Lett. 2022, 24, 642. |
| [3] | (f) Chen J.-Q.; Chen Q.; Chen B.; Wu J. Org. Chem. Front. 2023, 10, 2018. |
| [3] | (g) Hou H.; Cheng Y.; Chen B.; Tung C.; Wu L. Chin. J. Org. Chem. 2023, 43, 1012.. (in Chinese) |
| [3] | ( 侯虹宇, 程元元, 陈彬, 佟振合, 吴骊珠, 有机化学, 2023, 43, 1012.) |
| [4] | (a) Zheng X.; Dai X.-J.; Yuan H.-Q.; Ye C.-X.; Ma J.; Huang P.-Q. Angew. Chem. Int. Ed. 2013, 52, 3494. |
| [4] | (b) Xie H.; Guo J.; Wang Y.-Q.; Wang K.; Guo P.; Su P.-F.; Wang X.; Shu X.-Z. J. Am. Chem. Soc. 2020, 142, 16787. |
| [4] | (c) Pang X.; Su P.-F.; Shu X.-Z. Acc. Chem. Res. 2022, 55, 2491. |
| [5] | (a) Nguyen J. D.; Matsuura B. S.; Stephenson C. R. J. J. Am. Chem. Soc. 2014, 136, 1218. |
| [5] | (b) Nacsa E. D.; MacMillan D. W. C. J. Am. Chem. Soc. 2018, 140, 3322. |
| [5] | (c) Zhao G.; Yao W.; Mauro J. N.; Ngai M.-Y. J. Am. Chem. Soc. 2021, 143, 1728. |
| [6] | (a) Stache E. E.; Ertel A. B.; Rovis T.; Doyle A. G. ACS Catal. 2018, 8, 11134. |
| [6] | (b) Hu X.-Q.; Hou Y.-X.; Liu Z.-K.; Gao Y. Org. Chem. Front. 2020, 7, 2319. |
| [6] | (c) Shao X.; Zheng Y.; Ramadoss V.; Tian L.; Wang Y. Org. Biomol. Chem. 2020, 18, 5994. |
| [6] | (d) Guo H.-M.; Wu X. Nat. Commun. 2021, 12, 5365. |
| [7] | (a) Dong Z.; MacMillan D. W. C. Nature 2021, 598, 451. |
| [7] | (b) Sakai H. A.; MacMillan D. W. C. J. Am. Chem. Soc. 2022, 144, 6185. |
| [7] | (c) Intermaggio N. E.; Millet A.; Davis D. L.; MacMillan D. W. C. J. Am. Chem. Soc. 2022, 144, 11961. |
| [8] | (a) Barton D. H. R.; Crich D. Tetrahedron Lett. 1985, 26, 757. |
| [8] | (b) Barton D. H. R.; Crich D.; Kretzschmar G. J. Chem. Soc., Perkin Trans. 1 1986, 39. |
| [9] | Lackner G. L.; Quasdorf K. W.; Overman L. E. J. Am. Chem. Soc. 2013, 135, 15342. |
| [10] | Lackner G. L.; Quasdorf K. W.; Pratsch G.; Overman L. E. J. Org. Chem. 2015, 80, 6012. |
| [11] | Gao C.; Li J.; Yu J.; Yang H.; Fu H. Chem. Commun. 2016, 52, 7292. |
| [12] | Chen X.; Luo X.; Peng X.; Guo J.; Zai J.; Wang P. Chem. Eur. J. 2020, 26, 3226. |
| [13] | (a) Nawrat C. C.; Jamison C. R.; Slutskyy Y.; MacMillan D. W. C.; Overman L. E. J. Am. Chem. Soc. 2015, 137, 11270. |
| [13] | (b) Nawrat C. C.; Jamison C. R.; Slutskyy Y.; MacMillan D. W. C.; Overman L. E. J. Am. Chem. Soc. 2016, 138, 1724. |
| [14] | Lowry M. S.; Goldsmith J. L.; Slinker J. D.; Rohl R.; Pascal R. A.; Malliaras G. G.; Bernhard S. Chem. Mater. 2005, 17, 5712. |
| [15] | Slutskyy Y.; Jamison C. R.; Zhao P.; Lee J.; Rhee Y. H.; Overman L. E. J. Am. Chem. Soc. 2017, 139, 7192. |
| [16] | Garnsey M. R.; Slutskyy Y.; Jamison C. R.; Zhao P.; Lee J.; Rhee Y. H.; Overman L. E. J. Org. Chem. 2018, 83, 6958. |
| [17] | Allred T. K.; Dieskau A. P.; Zhao P.; Lackner G. L.; Overman L. E. Angew. Chem. Int. Ed. 2020, 59, 6268. |
| [18] | Allred T. K.; Dieskau A. P.; Zhao P.; Lackner G. L.; Overman L. E. J. Org. Chem. 2020, 85, 15532. |
| [19] | Abbas S. Y.; Zhao P.; Overman L. E. Org. Lett. 2018, 20, 868. |
| [20] | Pitre S. P.; Muuronen M.; Fishman D. A.; Overman L. E. ACS Catal. 2019, 9, 3413. |
| [21] | Dong J.; Wang Z.; Wang X.; Song H.; Liu Y.; Wang Q. J. Org. Chem. 2019, 84, 7532. |
| [22] | Lipp B.; Nauth A. M.; Opatz T. J. Org. Chem. 2016, 81, 6875. |
| [23] | Iwai K.; Takemura F.; Furue M.; Nozakura S.-I. Bull. Chem. Soc. Jpn. 1984, 57, 763. |
| [24] | Zuo Z.; MacMillan D. W. C. J. Am. Chem. Soc. 2014, 136, 5257. |
| [25] | Amos S. G. E.; Cavalli D.; Vaillant F. L.; Waser J. Angew. Chem. Int. Ed. 2021, 60, 23827. |
| [26] | Li M.; Liu T.; Li J.; He H.; Dai H.; Xie J. J. Org. Chem. 2021, 86, 12386. |
| [27] | Luo J.; Zhang J. ACS Catal. 2016, 6, 873. |
| [28] | Zhang X.; MacMillan D. W. C. J. Am. Chem. Soc. 2016, 138, 13862. |
| [29] | Guo L.; Song F.; Zhu S.; Li H.; Chu L. Nat. Commun. 2018, 9, 4543. |
| [30] | (a) Wang X.; Chen Y.; Liang P.; Chen J.-Q.; Wu J. Org. Chem. Front. 2022, 9, 4328. |
| [30] | (b) Chen M.; Sun W.; Yang J.; Yuan L.; Chen J.; Wu J. Green Chem. 2023, 25, 3857. |
| [31] | Guo L.; Tu H.-Y.; Zhu S.; Chu L. Org. Lett. 2019, 21, 4771. |
| [32] | Li H.; Guo L.; Feng X.; Huo L.; Zhu S.; Chu L. Chem. Sci. 2020, 11, 4904. |
| [33] | Su J. Y.; Gru?nenfelder D. C.; Takeuchi K.; Reisman S. E. Org. Lett. 2018, 20, 4912. |
| [34] | Brioche J. Tetrahedron Lett. 2018, 59, 4387. |
| [35] | Gonzalez-Esguevillas M.; Miró J.; Jeffrey J. L.; MacMillan D. W. C. Tetrehedron 2019, 75, 4222. |
| [36] | Troyano J. A.; Ballaschk F.; Jaschinski M.; ?zkaya Y.; Gómez-Suárez A. Chem. Eur. J. 2019, 25, 14054. |
| [37] | Vincent é.; Brioche J. Eur. J. Org. Chem. 2021, 2421. |
| [38] | Wang Q.; Yue L.; Bao Y.; Wang Y.; Kang D.; Gao Y.; Yuan Z. J. Org. Chem. 2022, 87, 8237. |
| [39] | Yan X. B.; Li C.-L.; Jin W.-J.; Guo P.; Shu X.-Z. Chem. Sci. 2018, 9, 4529. |
| [40] | Ye Y.; Chen H.; Sessler J. L.; Gong H. J. Am. Chem. Soc. 2019, 141, 820. |
| [41] | Gao M.; Sun D.; Gong H. Org. Lett. 2019, 21, 1645. |
| [42] | Ye Y.; Chen H.; Yao K.; Gong H. Org. Lett. 2020, 22, 2070. |
| [43] | Chen H.; Ye Y.; Tong W.; Fang J.; Gong H. Chem. Commun. 2020, 56, 454. |
| [44] | Ye Y.; Ma G.; Yao K.; Gong H. Synlett 2021, 32, 1625. |
| [45] | Friese F. W.; Studer A. Angew. Chem. Int. Ed. 2019, 58, 9561. |
| [46] | Ma G.; Chen C.; Talukdar S.; Zhao X.; Lei C.; Gong H. Chem. Commun. 2020, 56, 10219. |
| [47] | Guo P.; Wang K.; Jin W.-J.; Xie H.; Qi L.; Liu X.-Y.; Shu X.-Z. J. Am. Chem. Soc. 2021, 143, 513. |
| [48] | Chen Y.; Wang F.; Liu B.-X.; Rao W.-D.; Wang S.-Y. Org. Chem. Front. 2022, 9, 731. |
| [49] | Zhuo J.; Zhu C.; Wu J.; Li Z.; Li C. J. Am. Chem. Soc. 2022, 144, 99. |
/
| 〈 |
|
〉 |