

Investigation of the Kinetic Properties and Photoelectrochemical Water Splitting of Cu3V2O8/ZnO Photoanode Modified by Cobalt Phosphate
Received date: 2023-09-04
Online published: 2023-11-24
Supported by
National Natural Science Foundation of China(51976169)
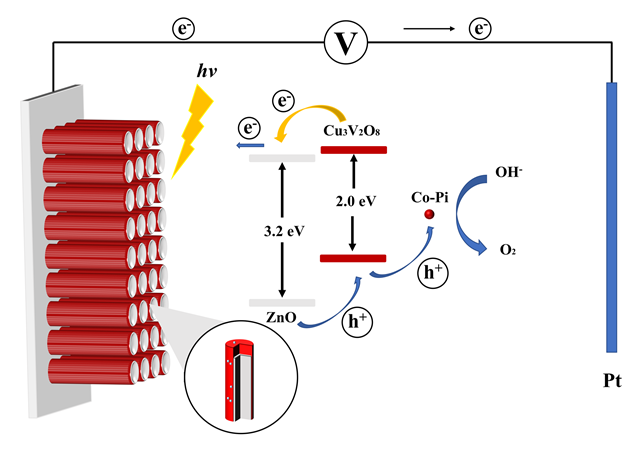
Copper vanadate (Cu3V2O8) has emerged as a promising material for photoelectrochemical applications due to its unique electronic and optical properties. In this study, the preparation of Cu3V2O8 photoelectrodes with different thicknesses was reported and the factors that affect their photoelectrochemical performance were investigated. It is found that the primary limitation of Cu3V2O8 as a photoanode material is the short diffusion distance of the photo-generated charge carriers, which leads to severe bulk recombination and limits the photocurrent density and efficiency. To overcome this limitation, a novel strategy of using one-dimensional ZnO nanorod arrays as a support framework and loading CoPi cocatalyst to enhance the photoelectrochemical performance of Cu3V2O8 was proposed. The ZnO nanorod arrays serve as a rapid electron transfer channel to promote the bulk separation of photo-generated charge carriers, while the CoPi cocatalyst improves the surface separation efficiency of photo-generated charge carriers and enhances the oxidation kinetics. The incorporation of ZnO nanorod arrays and CoPi cocatalyst significantly enhances the photocurrent density and efficiency of Cu3V2O8 photoelectrodes, resulting in a more than 2-fold increase in the photocurrent density and a 50% increase in the photoconversion efficiency. To further understand the underlying mechanisms behind the improved photoelectrochemical performance, a comprehensive analysis of the intensity-modulated photocurrent spectroscopy and electrochemical impedance spectroscopy data was performed. The results show that the incorporation of ZnO nanorod arrays and CoPi cocatalyst leads to a significant reduction in the charge transfer resistance and a decrease in the recombination rate of photo-generated charge carriers, which are responsible for the improved photocurrent density and efficiency. Overall, the study demonstrates the potential of using ZnO nanorod arrays and CoPi cocatalyst as a novel strategy to enhance the photoelectrochemical performance of Cu3V2O8 photoelectrodes. The proposed approach provides a new direction for the development of high-performance photoanode materials for solar energy conversion and other photoelectrochemical applications.
Zhiqiang Wang , Jinzhan Su . Investigation of the Kinetic Properties and Photoelectrochemical Water Splitting of Cu3V2O8/ZnO Photoanode Modified by Cobalt Phosphate[J]. Acta Chimica Sinica, 2024 , 82(1) : 26 -35 . DOI: 10.6023/A23090403
| [1] | Zhao, C.; Ma, Y.; Wang, Y.; Zhou, X.; Li, H.-Z.; Li, M.-Z.; Song, Y.-L. Acta Chim. Sinica 2018, 76, 9. (in Chinese) |
| [1] | (赵聪, 马颖, 汪洋, 周雪, 李会增, 李明珠, 宋延林, 化学学报, 2018, 76, 9.) |
| [2] | Liu, Y.; Li, Q.; Lian, Z.; Fan, J.; Tao, Y.; Li, G.; Li, H. Chem. Eng. J. 2022, 437, 135132. |
| [3] | Pirrone, N.; Bella, F.; Hernández, S. Green Chem. 2022, 24, 5379. |
| [4] | An, P.; Zhang, Q.-H.; Yang, Z.; Wu, J.-X.; Zhang, J.-Y.; Wang, Y.-J.; Li, Y.-M.; Jiang, G.-Y. Acta Chim. Sinica 2022, 80, 1629. (in Chinese) |
| [4] | (安攀, 张庆慧, 杨状, 武佳星, 张佳颖, 王雅君, 李宇明, 姜桂元, 化学学报, 2022, 80, 1629.) |
| [5] | Wang, J.; Wang, M.; Zhang, T.; Wang, Z.; Guo, P.; Su, J. ACS Appl. Mater. Interfaces 2018, 10, 12602. |
| [6] | Tayebi, M.; Tayyebi, A.; Masoumi, Z.; Lee, B.-K. Appl. Surf. Sci. 2020, 502, 144189. |
| [7] | Wang, J.; Perry, N. H.; Guo, L.; Vayssieres, L.; Tuller, H. L. ACS Appl. Mater. Interfaces 2018, 11, 2031. |
| [8] | Tayebi, M.; Kolaei, M.; Tayyebi, A.; Masoumi, Z.; Belbasi, Z.; Lee, B.-K. Sol. Energy. 2019, 190, 185. |
| [9] | Zhang, T.; Paulose, M.; Neupane, R.; Schaffer, L. A.; Rana, D. B.; Su, J.; Guo, L.; Varghese, O. K. Sol. Energy Mater. 2020, 209, 110472. |
| [10] | Hao, R.; Deng, X.; Yang, Y.-B.; Chen, D.-Y. Acta Chim. Sinica 2014, 72, 1199. (in Chinese) |
| [10] | (郝锐, 邓霄, 杨毅彪, 陈德勇, 化学学报, 2014, 72, 1199.) |
| [11] | Sivula, K.; Van De Krol, R. Nat. Rev. Mater. 2016, 1, 1. |
| [12] | Wang, Z.; Huang, X.; Wang, X. Catal. Today 2019, 335, 31. |
| [13] | Seabold, J. A.; Neale, N. R. Chem. Mater. 2015, 27, 1005. |
| [14] | Jiang, C.-M.; Farmand, M.; Wu, C. H.; Liu, Y.-S.; Guo, J.; Drisdell, W. S.; Cooper, J. K.; Sharp, I. D. Chem. Mater. 2017, 29, 3334. |
| [15] | Pulipaka, S.; Boni, N.; Meduri, P. ACS Appl. Energy Mater. 2020, 3, 6060. |
| [16] | Tahir, M.; Iqbal, T.; Zeba, I.; Hasan, A.; Muhammad, S.; Siddeeg, S. M.; Shahzad, K. J. Electrochem. Energy Convers. Storage. 2020, 17, 011002. |
| [17] | Lian, X.; Duan, H.; Zeng, W.; Yu, B.; Guo, W.; Lou, Q. Mol. Catal. 2022, 528, 112493. |
| [18] | Fujimoto, I.; Wang, N.; Saito, R.; Miseki, Y.; Gunji, T.; Sayama, K. Int. J. Hydrogen Energy 2014, 39, 2454. |
| [19] | Hossain, M. K.; Sarker, H. P.; Sotelo, P.; Dang, U.; Rodriguez-Gutierrez, I.; Blawat, J.; Vali, A.; Xie, W.; Oskam, G.; Huda, M. N. Chem. Mater. 2020, 32, 6247. |
| [20] | He, X.; Gan, J.; Li, H. J. Taiwan Inst. Chem. Eng. 2021, 127, 119. |
| [21] | Hong, S.; Burkhow, S. J.; Doughty, R. M.; Cheng, Y.; Ryan, B. J.; Mantravadi, A.; Roling, L. T.; Panthani, M. G.; Osterloh, F. E.; Smith, E. A. Chem. Mater. 2021, 33, 1667. |
| [22] | Liu, C.; Su, J.; Guo, L. RSC Adv. 2016, 6, 27557. |
| [23] | Chai, H.; Gao, L.; Wang, P.; Li, F.; Hu, G.; Jin, J. Appl. Catal., B 2022, 305, 121011. |
| [24] | Mana, P. M.; Bhujbal, P. K.; Pathan, H. M. Energy Environ. 2020, 12, 77. |
| [25] | Bertoluzzi, L.; Lopez-Varo, P.; Tejada, J.; Bisquert, J. J. Mater. Chem. A 2016, 4, 2873. |
| [26] | Sang, L.-X.; Lin, J.; Ge, H.; Lei, L. Acta Physico-Chimica Sinica 2017, 33, 2454. (in Chinese) |
| [26] | (桑丽霞, 蔺佳, 葛昊, 雷蕾, 物理化学学报, 2017, 33, 2454.) |
| [27] | Franco, G.; Gehring, J.; Peter, L.; Ponomarev, E.; Uhlendorf, I. J. Phys. Chem. B 1999, 103, 692. |
| [28] | Zhang, H.; Jin, S.; Duan, G.; Wang, J.; Cai, W. J. Mater. Sci. Technol. 2014, 30, 1118. |
| [29] | Kumar, V.; Gupta, R.; Bansal, A. ACS Appl. Nano Mater. 2021, 4, 6212. |
| [30] | Wei, Y.; Su, J.; Wan, X.; Guo, L.; Vayssieres, L. Nano Res. 2016, 9, 1561. |
| [31] | Tieu, D. T.; Trang, T. N. Q.; Thu, V. T. H. J. Alloys Compd. 2019, 808, 151735. |
| [32] | Doan, Q. K.; Nguyen, M. H.; Sai, C. D.; Mai, H. H.; Pham, N. H.; Bach, T. C.; Nguyen, V. T.; Nguyen, T. T.; Ho, K. H.; Tran, T. H. Appl. Surf. Sci. 2020, 505, 144593. |
| [33] | Fang, W.; Lin, Y.; Xv, R.; Shang, X.; Fu, L. Electrochim. Acta 2023, 437, 141511. |
| [34] | Balachandran, S.; Swaminathan, M. J. Phys. Chem. C 2012, 116, 26306. |
| [35] | Jin, T.; Xu, D.; Diao, P.; Xiang, M. Acta Physico-Chimica Sinica 2012, 28, 2276. (in Chinese) |
| [35] | (金涛, 许頔, 刁鹏, 项民, 物理化学学报, 2012, 28, 2276.) |
| [36] | Kim, J. Y.; Jang, J. W.; Youn, D. H.; Magesh, G.; Lee, J. S. Adv. Energy Mater. 2014, 4, 1400476. |
| [37] | Chen, Y.-C.; Wu, Z.-J.; Hsu, Y.-K. Nanoscale 2020, 12, 12292. |
/
| 〈 |
|
〉 |