

Synthesis of N-Benzylidene Benzylamine from Dibenzylamine and Derivatization under Electrochemical Conditions
Received date: 2024-04-07
Online published: 2024-05-08
Supported by
National Natural Science Foundation of China(22161046); Natural Science Foundation of Xinjiang Uygur Autonomous Region of China(2022D01A207)
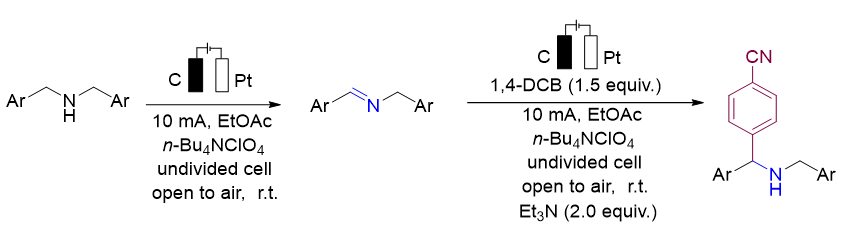
N-benzylidene benzylamine and its derivatives are common functional molecules in organic intermediates, fine chemicals and pharmaceuticals, and have been widely used in medicinal chemistry, materials science and natural product synthesis, such as the synthesis of anticancer and anti-inflammatory agents. Electrochemistry has become a powerful tool in organic synthesis to avoid the use of expensive and toxic oxidants or reductants to reduce the production of harmful and toxic by-products. Therefore, In this paper, we report a method for the synthesis of N-benzylidene benzylamine from dibenzylamine under electrochemical conditions, and its derivatization is studied by a one-pot tandem reaction. N-benzylidene benzylamines were electrolyzed at room temperature with graphite rod (Φ=6 mm) and Pt plate (10 mm×10 mm×0.2 mm) as electrode, ethyl acetate as solvent, and tetrabutylammonium perchlorate (n-Bu4NClO4) as electrolyte. Sixteen N-benzylidene benzylamines compounds were successfully prepared by this method, and the GC yield was as high as 96%. The “one-pot method” tandem reaction is a simple, fast and environment-friendly synthesis and preparation method, which uses simple, easy-to-obtain and inexpensive raw materials to prepare complex structures and high-value organic compounds through this “one-pot method” series reaction, without the separation and purification of intermediate products to obtain the required products, especially suitable for reaction systems with unstable intermediate products, which has the advantages of simple operation, simple post-treatment, less product loss, and less waste. In order to highlight the application value of N-benzylidene benzylamines, the derivatization reaction of N-benzylidene benzylamine by one-pot tandem method was further explored. And 15 derivatization products were prepared, isolated yield up to 86%. Finally, through control experiments, salt bridge experiments, cyclic voltammetry experiments and related literature reports, we speculated the possible reaction mechanism of this reaction. The reaction has the characteristics of mild conditions, wide range of substrates, good tolerance of functional groups, without redox agents and insensitivity to air and moisture. This study provides an efficient and economical synthesis strategy for the preparation of N-benzylidene benzylamines derivatives.
Yunusi Reziguli , Abuduwaili Kadierya , Shiwei Luo , Abudu Rexit Abulikemu . Synthesis of N-Benzylidene Benzylamine from Dibenzylamine and Derivatization under Electrochemical Conditions[J]. Acta Chimica Sinica, 2024 , 82(8) : 843 -848 . DOI: 10.6023/A24040119
| [1] | Huang, B.; Tian, H.; Lin, S.; Xie, M. H.; Yu, X. C.; Xu, Q. Tetrahedron Lett. 2013, 54, 2861. |
| [2] | Kobayashi, S.; Mori, Y.; Fossey, J. S.; Salter, M. M. Chem. Rev. 2011, 111, 2626. |
| [3] | (a) Bottaro, F.; Takallou, A.; Chehaiber, A.; Madsen, R. Eur. J. Org. Chem. 2019, 7164. |
| [3] | (b) Li, Y.; Shang, S.; Wang, L.; Lv, Y.; Niu, J.; Gao, S. Chem. Commun. 2019, 55, 12251. |
| [3] | (c) Gao, K.; Li, H.; Meng, Q.; Jie, Wu.; Hou, H. W. ACS Appl. Mater. 2021, 13, 2779. |
| [3] | (d) Chen, B.; Shang, S. S.; Wang, L. Y.; Zhang, Y.; Gao, S. Chem. Commun. 2015, 52, 481. |
| [3] | (e) Murray, A. T.; Dowley, M. J.; Pradaux-Caggiano, F.; Baldansuren, A.; Fielding, A. J.; Tuna, F.; Carbery, D. R. Angew. Chem., Int. Ed. 2015, 54, 8997. |
| [3] | (f) Monopoli, A.; Cotugno, P.; Iannone, F.; Ciminale, F.; Dell'Anna, M. M.; Mastrorilli, P.; Nacci, A. Eur. J. Org. Chem. 2014, 5925. |
| [3] | (g) Huang, H.; Huang, J.; Liu, Y. M.; He, H. Y.; Cao, Y.; Fan, K. N. Green Chem. 2012, 14, 930. |
| [3] | (h) Sobhani, S.; Maleki, M. Synlett. 2010, 0383. |
| [3] | (i) Kumar, I.; Kumar, R.; Gupta, S. S.; Sharma, U. J. Org. Chem. 2021, 86, 6449. |
| [4] | Choi, H.; Doyle, M. P. Chem. Commun. 2007, 745. |
| [5] | Chen, R.; Shi, J. L.; Ma, Y.; Lin, G.; Lang, X.; Wang, C. Angew. Chem., Int. Ed. 2019, 58, 6107. |
| [6] | Cyniak, J. S.; Kasprzak, A. J. Org. Chem. 2021, 86, 6855. |
| [7] | (a) Xie, J. Q.; Jia, Y. X. Chin. J. Org. Chem. 2023, 43, 2256 (in Chinese). |
| [7] | (谢佳琪, 贾义霞, 有机化学, 2023, 43, 2256.) |
| [7] | (b) Fan, Y.; Ou, W.; Chen, M. Y.; Liu, Y. B.; Zhang, B.; Ruan, W. Q.; Su, C. L. Org. Lett. 2023, 25, 432. |
| [8] | (a) McNally, A.; Prier, C. K.; MacMillan, D. W. Science 2011, 334, 1114. |
| [8] | (b) Ma, Y.; Yao, X.; Zhang, L.; Ni, P.; Cheng, R.; Ye, J. Angew. Chem., Int. Ed. 2019, 58, 16548. |
| [8] | (c) Xu, C.; Shen, F. Q.; Feng, G. F.; Jin, J. Org. Lett. 2021, 23, 3913. |
| [9] | (a) Chen, M.; Zhao, X.; Yang, C.; Xia, W. Org. Lett. 2017, 19, 14. |
| [9] | (b) Zeng, W. M.; He, Y. H.; Guan, Z. Org. Lett. 2022, 24, 39. |
| [10] | (a) Tang, M.; Wu, Y.; Liu, Y.; Cai, M. Q.; Xia, F.; Liu, S. Y.; Hu, W. H. Acta Chim. Sinica 2016, 74, 54 (in Chinese). |
| [10] | (唐敏, 吴永, 刘源, 蔡茂强, 夏飞, 刘顺英, 胡文浩, 化学学报, 2016, 74, 54.); |
| [10] | (b) Tong, T.; Wu, X.; Li, E. F.; Kang, H. L.; Wang, X. X.; Lv, X. T. J. Org. Chem. 2018, 83, 15533. |
| [10] | (c) Wang, H. L.; Yu, Q. X.; Zhu, Z. H.; Lu, X. L.; Li, Y. L.; Liang, F. P.; Zou, H. H. Inorg. Chem. 2023, 62, 5863. |
| [11] | (a) Feng, E. Q.; Hou, Z. W.; Xu, H. C. Chin. J. Org. Chem. 2019, 39, 1424 (in Chinese). |
| [11] | (冯恩祺, 侯中伟, 徐海超, 有机化学, 2019, 39, 1424). |
| [11] | (b) Chen, N.; Lai, X. L.; Xu, H. C. Chin. J. Org. Chem. 2020, 40, 2592 (in Chinese). |
| [11] | (陈娜, 赖小丽, 徐海超, 有机化学, 2020, 40, 2592). |
| [11] | (c) Abudulajiang, N.; Chang, X.; Guo, C. J. Org. Chem. 2021, 86, 16068. |
| [11] | (d) Wang, Z. H.; Ma, C.; Fang, P.; Xu, H. C.; Mei, T. S. Acta Chim. Sinica 2022, 80, 1115 (in Chinese). |
| [11] | (王振华, 马聪, 方萍, 徐海超, 梅天胜, 化学学报, 2022, 80, 1115.). |
| [11] | (e) Gao, R. L.; Wen, L. R.; Guo, W. S. Chin. J. Org. Chem. 2024, 44, 892 (in Chinese). |
| [11] | (高瑞林, 文丽荣, 郭维斯, 有机化学, 2024, 44, 892.) |
| [11] | (f) Aman, H.; Chang, R.; Ye, J. T. Chin. J. Org. Chem. 2024, 44, 728 (in Chinese). |
| [11] | (Hasil, Aman, 常瑞, 叶俊涛, 有机化学, 2024, 44, 728.) |
| [12] | (a) Maidina, S.; Naibijiang, S.; Luo, S. W.; Mailikezhati, M.; Rexit, A. A. J. Org. Chem. 2024, 89, 3696. |
| [12] | (b) Kanbinuer, N.; Wang, C.; Luo, S. W.; Rexit, A. A. Acta Chim. Sinica 2023, 81, 582 (in Chinese). |
| [12] | (坎比努尔?努尔买买提, 王超, 罗时玮, 阿布都热西提?阿布力克木, 化学学报, 2023, 81, 582.) |
| [13] | Jiang, W. X.; Liu, X.; Zhu, C. Y.; Chen, M. Y.; Li, W. D.; Cao, H. Org. Chem. Front. 2024, 11, 2306. |
/
| 〈 |
|
〉 |