

Studies on the Properties of 1,5-Diaminoanthraquinone (AAQ) Composite Used as New Positive Electrode Material in Lithium Ion Batteries
Received date: 2024-02-06
Online published: 2024-05-13
Supported by
National Natural Science Foundation of China(51972330); National Key Research and Development Program of China(2022YFB3704700); National Key Research and Development Program of China(2022YFB3704702); Major Scientific and Technological Innovation Project of Shandong Province(2021CXGC010901); Taishan Scholar Program of Shandong Province(TS201511031); Education Department of Shandong Province(SDYJG19089)
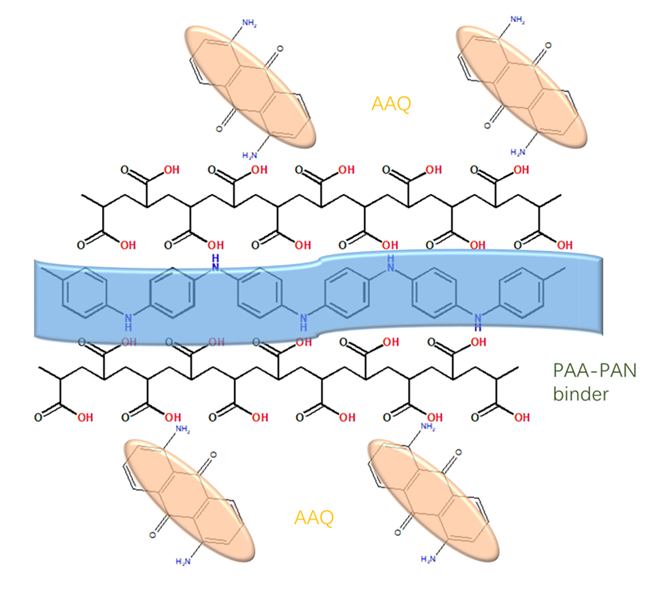
Organic conjugated small molecular material 1,5-diaminoanthraquinone (AAQ), as an organic cathode material for lithium-ion battery, has become the research focus of new energy battery materials because of its simple preparation, high theoretical specific capacity and stable cycle performance. However, due to its poor conductivity and dissolution in electrolyte, its electrochemical properties are affected, which limits its further application. In this work, AAQ was dispersed in metal organic framework porous materials (MOFs) - Nitrogen-doped porous carbon (ZIF-8C) by ultrasonic method, and then porous carbon framework composites AAQ@ZIF-8C were prepared by heat-treatment method. Copolymer PAA-PAN was prepared by solution polymerization method with polyacrylic acid (PAA) and aniline (AN) monomer. Finally, AAQ@ZIF-8C/ PAA-PAN composite electrode material were prepared with above materials by ball milling method. The structure, morphology and electrochemical properties of the composite electrode materials were characterized by infrared spectroscopy, scanning electron microscopy, electrochemical cyclic voltammetry and AC impedance method. When PAA-PAN combined with AAQ@ZIF-8C, the conductive interpenetrating network structure is formed in AAQ@ZIF-8C/PAA-PAN composite, which not only improves the conductivity of the electrode active material AAQ, but also inhibits the solubility of AAQ in the electrolyte. In addition, the porous ZIF-8C skeleton material is conducive to the rapid diffusion and migration of electrolyte ions and improves the reversible capacity of the battery. High solubility in aprotic organic electrolytes and poor electrical conductivity are the main restrictions of organic electrodes in practical application. Conductive binder contributes to the high-performance electrodes as it enables both mechanical and electronic integrity of the electrode, which have been scarcely explored for organic electrodes. As we know, state-of-the-art lithium-ion battery (LIB) electrodes choose polyvinylidene fluoride (PVDF) as the binder because of its good chemical and electrochemical stability. Herein, a conductive interpenetrating polymeric network is synthesized through in situ polymerization of polyaniline with poly(acrylic acid) (denoted PAA-PAN), which served as a novel conductive binder for organic AAQ materials. The PAA was chosen as the matrix component for the reason that PAA contains sufficient carboxyl groups (-COOH), which can condense with the amino groups (-NH2) of polyaniline. Therefore, a conductive interpenetrating network can be formed in the process of in-situ polymerization. The conductive PAN component enhances the electrical conductivity of the electrode. Meanwhile, the PAA component serves as the binding matrix to condense with the amino groups (-NH2) of AAQ, which therefore effectively inhibits their dissolution and maintains electrode integrity during cycling. The electrochemical results showed that the lithium ion battery assembled with the composite electrode material has a reversible capacity of 203 mAh•g-1 at a Coulomb rate of 0.1 C, which is close to its theoretical specific capacity of 225 mAh•g-1. After 200 cycles, the reversible capacity is still 195 mAh•g-1 and the coulomb efficiency is 95%.
Zhucheng Wang , Lei Liu , Mengyuan Zhu , Yue Sun , Qing Zhao , Yuyin Ding , Jixin Lu , Cunguo Wang , Qi Li , Aihua He , Fuchen Ye . Studies on the Properties of 1,5-Diaminoanthraquinone (AAQ) Composite Used as New Positive Electrode Material in Lithium Ion Batteries[J]. Acta Chimica Sinica, 2024 , 82(6) : 589 -595 . DOI: 10.6023/A24020048
| [1] | (a) Levi, M. D.; Wang, C.; Gnanaraj, J.; Aurbach, D. J. Power Sources 2003, 119, 538. |
| [1] | (b) Goodenough, J. B.; Park, K.-S. J. Am. Chem. Soc. 2013, 135, 1167. |
| [1] | (c) Zhang, L.; Zhang, M.; Wang, Y.; Zhang, Z.; Kan, G.; Wang, C.; Zhong, Z.; Su, F. J. Mater. Chem. A 2014, 2, 10161. |
| [1] | (d) Liu, X.; Liu, X.; Sun, B.; Zhou, H.; Fu, A.; Wang, Y.; Guo, Y.-G.; Guo, P.; Li, H. Carbon 2018, 130, 680. |
| [1] | (e) Wang, C.; Song, H.; Yu, C.; Ullah, Z.; Guan, Z.; Chu, R.; Zhang, Y.; Zhao, L.; Li, Q.; Liu, L. J. Mater. Chem. A 2020, 8, 3421. |
| [1] | (f) Zhao, L. Y.; Sun, Y.; Zhao, Q.; Ullah, Z.; Zhu, S. P.; Zhu, M. Y.; Liu, L. W.; Wang, C. G.; Li, Q.; He, A. H.; Wang, Y. L.; Ye, F. C. Chem. Eng. J. 2024, 490, 151547. |
| [1] | (g) Bai, Y.; Wang, C.; Li, X.; Fan, W.; Song, P.; Gu, Y.; Liu, F.; Liu, G. Chem. J. Chinese U. 2020, 41, 1306. |
| [2] | (a) Liu, Z.; Hu, F.; Xiang, J.; Yue, C.; Lee, D.; Song, T. Part. Part. Syst. Char. 2018, 35, 1800163. |
| [2] | (b) Li, W.; Dolocan, A.; Oh, P.; Celio, H.; Park, S.; Cho, J.; Manthiram, A. Nat. Commun. 2017, 8, 14589. |
| [2] | (c) Yang, Y.; Zheng, X.; Cao, H.; Zhao, C.; Lin, X.; Ning, P.; Zhang, Y.; Jin, W.; Sun, Z. ACS Sustain. Chem. Eng. 2017, 5, 9972. |
| [2] | (d) Liu, W.; Oh, P.; Liu, X.; Lee, M. J.; Cho, W.; Chae, S.; Kim, Y.; Cho, J. Angew. Chem. Int. Ed. 2015, 54, 4440. |
| [3] | Shi, T.; Chen, J.; Zheng, J.; Li, X.; Zhou, B.; Cao, H.; Wang, Y. Sci. Rep. 2016, 6, 39132. |
| [4] | (a) Guo, S.; Li, Q.; Liu, P.; Chen, M.; Zhou, H. Nat. Commun. 2017, 8, 135. |
| [4] | (b) Lee, M.; Hong, J.; Kim, H.; Lim, H. D.; Cho, S. B.; Kang, K.; Park, C. B. Adv. Mater. 2014, 26, 2558. |
| [5] | (a) Deng, W.; Yu, J.; Qian, Y.; Wang, R.; Ullah, Z.; Zhu, S.; Chen, M.; Li, W.; Guo, Y.; Li, Q. Electrochim. Acta 2018, 282, 24. |
| [5] | (b) Miroshnikov, M.; Divya, K. P.; Babu, G.; Meiyazhagan, A.; Arava, L. M. R.; Ajayan, P. M.; John, G. J. Mater. Chem. A 2016, 4, 12370. |
| [5] | (c) Zhang, Y.; Huang, Y.; Yang, G.; Bu, F.; Li, K.; Shakir, I.; Xu, Y. ACS Appl. Mater. Interfaces 2017, 9, 15549. |
| [5] | (d) Fan, W. Q.; Chu, R. R.; Wang, C. G.; Song, H. W.; Ding, Y. Y.; Li, X.; Jiang, M. Y.; Li, Q.; Liu, L. W.; He, A. H. Compos. Part B-Eng. 2021, 226, 109329. |
| [6] | (a) Wang, J.; Yang, J.; Xie, J.; Xu, N. Adv. Mater. 2002, 14, 963. |
| [6] | (b) Song, Z.; Qian, Y.; Gordin, M. L.; Tang, D.; Xu, T.; Otani, M.; Zhan, H.; Zhou, H.; Wang, D. Angew. Chem. Int. Ed. 2015, 54, 13947. |
| [7] | Jiao, X.; Teng, C.; Li, C. P.; Wei, Z. D. J. Chem. Ind. Eng. 2023, 74, 170. (in Chinese) |
| [7] | (焦巡, 童成, 李存璞, 魏子栋, 化工学报, 2023, 74, 170.) |
| [8] | (a) Luo, R.; Wang, C.; Zhang, Z.; Lv, W.; Wei, Z.; Zhang, Y.; Luo, X.; He, W. ACS Appl. Energy Mater. 2018, 1, 921. |
| [8] | (b) Wu, H.; Wang, K.; Meng, Y.; Lu, K.; Wei, Z. J. Mater. Chem. A 2013, 1, 6366. |
| [9] | Zhang, J.; Huang, L. Y.; Cai, F. S.; Luo, Z. Q.; Yuan, Z. H. Chinese J. Eng. 2023, 45, 1165. (in Chinese) |
| [9] | (张瑾, 黄莉雅, 蔡锋石, 罗志强, 袁志好, 工程科学学报, 2023, 45, 1165.) |
| [10] | Zhang, K.; Guo, C.; Zhao, Q.; Niu, Z.; Chen, J. Adv. Sci. 2015, 2, 1500018. |
| [11] | (a) Zhao, L.; Yu, J.; Xing, C.; Ullah, Z.; Yu, C.; Zhu, S.; Chen, M.; Li, W.; Li, Q.; Liu, L. Energy Storage Mater. 2019, 22, 433. |
| [11] | (b) Shen, K.; Zhang, L.; Chen, X.; Liu, L.; Zhang, D.; Han, Y.; Chen, J.; Long, J.; Luque, R.; Li, Y. Science 2018, 359, 206. |
| [11] | (c) Zhu, P.; Ullah, Z.; Zheng, S.; Yang, Z.; Yu, S.; Zhu, S.; Liu, L.; He, A.; Wang, C.; Li, Q. Nano Energy 2023, 108, 108195. |
| [12] | (a) Zhao, L.; Wang, W.; Wang, A.; Yuan, K.; Chen, S.; Yang, Y. J. Power Sources 2013, 233, 23. |
| [12] | (b) Song, P.; Zheng, S.; Ullah, Z.; Yang, Z.; Zhu, P.; He, A.; Wang, C.; Li, Q. ACS Appl. Energy Mater. 2023, 6, 4671. |
| [12] | (c) Song, J.; Gordin, M. L.; Xu, T.; Chen, S.; Yu, Z.; Sohn, H.; Lu, J.; Ren, Y.; Duan, Y.; Wang, D. Angew. Chem. Int. Ed. 2015, 54, 4325. |
| [13] | Zhao, L.; Wang, A. B.; Wang, W. K.; Yu, Z. B.; Chen, S.; Yang, Y. S. Acta Phys.-Chim. Sin. 2012, 28, 596. (in Chinese) |
| [13] | (赵磊, 王安邦, 王维坤, 余仲宝, 陈实, 杨裕生, 物理化学学报, 2012, 28, 596.) |
| [14] | (a) Yu, X.; Yang, H.; Meng, H.; Sun, Y.; Zheng, J.; Ma, D.; Xu, X. ACS Appl. Mater. Interfaces 2015, 7, 15961. |
| [14] | (b) Yan, L.; Gao, X.; Thomas, J. P.; Ngai, J.; Altounian, H.; Leung, K. T.; Meng, Y.; Li, Y. Sustain. Energ. Fuels 2018, 2, 1574. |
| [15] | Tong, J.; Han, C.; Hao, X.; Qin, X.; Li, B. ACS Appl. Mater. Interfaces 2020, 12, 39630. |
| [16] | Martín-Jimeno, F. J.; Suarez-García, F.; Paredes, J. I.; Enterría, M.; Pereira, M. F. R.; Martins, J. I.; Figueiredo, J. L.; Martínez-Alonso, A.; Tascón, J. M. D. ACS Appl. Mater. Interfaces 2017, 9, 44740. |
/
| 〈 |
|
〉 |