

Solvent-free Oxidative Dehydrogenation N—N Coupling Reaction of N-Alkoxyamides
Received date: 2024-03-23
Online published: 2024-05-14
Supported by
National Natural Science Foundation of China(22161046)
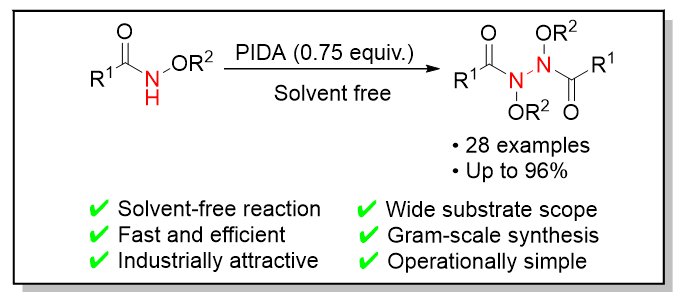
Organic synthesis usually requires organic solvents to dissolve reactants or catalysts for reaction, but this leads to serious waste of solvents and environmental pollution. The solvent-free organic synthesis reaction method can effectively avoid resource waste and environmental pollution caused by the use of solvents, and without the use of solvents, the concentration of reactants is the highest, it has significant advantages over reactions in solution in terms of reaction speed, reaction operation, yield, etc. Therefore, solvent-free organic synthesis reaction is favored by organic synthesis chemists as a cleaner and more sustainable synthesis reaction method. However, skeleton structures containing N—N bonds are a very important class of structural units, such as azo compounds, hydrazine derivatives, pyrazole, indazole, triazole derivatives, etc. that exist in various natural products, functional drug molecules, and organic materials. Currently, more than 300 drug molecules have been reported to contain N—N bonds. Especially N,N-disubstituted hydrazine has a wide range of physiological and pharmacological activities, such as antihypertensive, anticancer, and antibacterial. Therefore, we reported using iodobenzenediacetic acid (PIDA) as an oxidant, N-alkoxyamide compounds underwent N—N self coupling reaction at room temperature and solvent-free conditions. This method not only has a simple and easy to operate system, high reaction efficiency, and can prepare products at the gram level, but also has good functional group compatibility, suitable for various substituted benzene rings, aromatic heterocycles, and aliphatic amide substrates. This provides a simple, mild, efficient and potentially useful preparation method for N—N self-coupling of N-alkoxyamide compounds with different substituents. Under this condition, 28 coupling products were prepared with a maximum yield of 96%. The typical procedure is as follows: the mixture of N-alkoxyamide (0.2 mmol, 1 equiv.) and oxidant PIDA (0.15 mmol, 0.75 equiv.) is stirred under a magnetic stirrer until the starting material is completely consumed under thin layer chromatography monitoring. After the reaction, the N—N coupling product was purified by silica gel chromatography.
Abuduwaili Kadierya , Yunusi Reziguli , Jiajia Li , Shiwei Luo , Abudu Rexit Abulikemu . Solvent-free Oxidative Dehydrogenation N—N Coupling Reaction of N-Alkoxyamides[J]. Acta Chimica Sinica, 2024 , 82(7) : 731 -735 . DOI: 10.6023/A24030098
| [1] | Constable D. J. C.; Jimenez-Gonzalez C.; Henderson R. K. Org. Process Res. Dev. 2007, 11, 133. |
| [2] | Ouyang J. S.; Zhang X.; Pan B.; Zou H.; Chan A. S.; Qiu L. Org. Lett. 2023, 25, 7491. |
| [3] | Zang H. J.; Li Z. M.; Wang B. L. Chin. J. Org. Chem. 2003, 23, 1058 (in Chinese). |
| [3] | (臧洪俊, 李正名, 王宝雷, 有机化学, 2003, 23, 1058.) |
| [4] | (a) Mao Z. C.; Chen J. R. Chin. J. Org. Chem. 2023, 43, 4314 (in Chinese). |
| [4] | (毛志诚, 陈加荣, 有机化学, 2023, 43, 4314.) |
| [4] | (b) Yang M.; Ye B. B.; Chen J. Q.; Wu J. Acta Chim. Sinica 2022, 80, 11 (in Chinese). |
| [4] | (杨民, 叶柏柏, 陈健强, 吴劼, 化学学报, 2022, 80, 11.) |
| [4] | (c) Ma H. J.; Zhou F. Y.; Liu J. L.; Han B.; Yang H.; Zhang Y. Q.; Wang J. J. Chin. J. Org. Chem. 2022, 42, 1843. (in Chinese). |
| [4] | (马豪杰, 周风院, 刘金磊, 韩波, 杨华, 张玉琦, 王记江, 有机化学, 2022, 42, 1843.) |
| [4] | (d) Jing Z. X.; Du J. X.; Jiang P.; Ablajan K. Chin. J. Org. Chem. 2023, 43, 3930. (in Chinese). |
| [4] | (景智霞, 杜建喜, 蒋平, 阿布拉江?克依木, 有机化学, 2023, 43, 3930.) |
| [4] | (e) Liu X.; Kuang C. X.; Su C. H. Acta Chim. Sinica 2022, 80, 1135 (in Chinese). |
| [4] | (刘霞, 匡春香, 苏长会, 化学学报, 2022, 80, 1135.) |
| [5] | Blair L. M.; Sperry J. J. Nat. Prod. 2013, 76, 794. |
| [6] | (a) Zhang R.; Durkin J. P.; Windsor W. T. Bioorg. Med. Chem. Lett. 2002, 12, 1005. |
| [6] | (b) Zhou C.-H.; Wang Y. Curr. Med. Chem. 2012, 19, 239. |
| [6] | (c) Kü?ükgüzel ?. G.; ?enkarde? S. Eur. J. Med. Chem. 2015, 97, 786. |
| [7] | (a) Merino E. Chem. Soc. Rev. 2011, 40, 3835. |
| [7] | (b) Maleix C.; Chabernaud P.; Brahmi R. Acta Astronautica 2019, 158, 407. |
| [8] | Ke S. Y.; Sun T. T.; Liang Y.; Yang Z. W. Chin. J. Org. Chem. 2010, 30, 1820 (in Chinese). |
| [8] | (柯少勇, 孙婷婷, 梁英, 杨自文, 有机化学, 2010, 30, 1820.) |
| [9] | Lemke T. L.; Sanduja R.; Mroue M. M.; Iyer S.; Alam M.; Hossain M. B.; van der Helm D. J. Pharm. Sci. 1990, 79, 840. |
| [10] | (a) Shoeb M.; MacManus S. M.; Jaspars M.; Trevidu J.; Nahar L.; Kong-Thoo-Lin P.; Sarker S. D. Tetrahedron 2006, 62, 11172. |
| [10] | (b) Ueberschaar N.; Ndejouong B. L.; Ding L.; Maier A.; Fiebig H. H.; Hertweck C. Bioorg. Med. Chem. Lett. 2011, 21, 5839. |
| [11] | Raju B.; Mortell K.; Anandan S.; O'Dowd H.; Gao H.; Gomez M.; Hackbarth C.; Wu C.; Wang W.; Yuan Z.; White R. Bioorg. Med. Chem. Lett. 2003, 13, 2413. |
| [12] | Cahn J. W.; Powell R. E. J. Am. Chem. Soc. 1954, 76, 2565. |
| [13] | Perkin W. H.; Tucker S. H. J. Chem. Soc. 1921, 119, 216. |
| [14] | Branch G. E. K.; Smith J. F. J. Am. Chem. Soc. 1920, 42, 2405. |
| [15] | McLintock J.; Tucker S. H. J. Chem. Soc. 1927, 1214. |
| [16] | (a) Yan X.-M.; Chen Z.-M.; Yang F.; Huang Z.-Z. Synlett 2011, 2011, 569. |
| [16] | (b) Zhu Y.; Shi Y. Org. Lett. 2013, 15, 1942. |
| [16] | (c) Reddy C. B. R.; Reddy S. R.; Naidu S. Catal. Commun. 2014, 56, 50. |
| [16] | (d) Fritsche R. F.; Theumer G.; Kataeva O.; Kn?lker H.-J. Angew. Chem. Int. Ed. 2017, 56, 549. |
| [16] | (e) Ryan M. C.; Martinelli J. R.; Stahl S. S. J. Am. Chem. Soc. 2018, 140, 9074. |
| [16] | (f) Wang H.; Jung H.; Song F.; Zhu S.; Bai Z.; Chen D.; He G.; Chang S.; Chen G. Nat. Chem. 2021, 13, 378. |
| [16] | (g) Song F. F.; Zhu S. Y.; Wang H.; Chen G. Chin. J. Org. Chem. 2021, 41, 4050. (in Chinese). |
| [16] | (宋方方, 朱士阳, 王浩, 陈弓, 有机化学, 2021, 41, 4050.) |
| [16] | (h) Barbor J. P.; Nair V. N.; Sharp K, R.; Lohrey T. D.; Dibrell S. E.; Shah T. K.; Walsh M. J.; Reisman S. E.; Stoltz B. M. J. Am. Chem. Soc. 2023, 145, 15071. |
| [16] | (i) Zhu S. Y.; He W. J.; Shen G. C.; Bai Z. Q.; Song F. F.; He G.; Chen G. Angew. Chem. Int. Ed. 2024, 63, e202312465. |
| [17] | (a) Gieshoff T.; Kehl A.; Schollmeyer D.; Moeller K. D.; Waldvogel S. R. J. Am. Chem. Soc. 2017, 139, 12317. |
| [17] | (b) Kehl A.; Gieshoff T.; Schollmeyer D.; Waldvogel S. R. Chem. Eur. J. 2018, 24, 590. |
| [17] | (c) Feng E. Q.; Hou Z. W.; Xu H. C. Chin. J. Org. Chem. 2019, 39, 1424 (in Chinese). |
| [17] | (冯恩祺, 侯中伟, 徐海超, 有机化学, 2019, 39, 1424). |
| [17] | (d) Breising V. M.; Kayser J. M.; Kehl A.; Schollmeyer D.; Liermann J. C.; Waldvogel S. R. Chem. Commun. 2020, 56, 4348. |
| [17] | (e) Lv S.; Han X.; Wang J.-Y.; Zhou M.; Wu Y.; Ma L.; Niu L.; Gao W.; Zhou J.; Hu W.; Cui Y.; Chen J. Angew. Chem. Int. Ed. 2020, 59, 11583. |
| [18] | Nasier A.; Chang X.; Guo C. J. Org. Chem. 2021, 86, 16068. |
| [19] | Subramanian K.; Yedage S. L.; Bhanage B. M. Adv. Synth. Catal. 2018, 360, 2511. |
| [20] | (a) Parra A.; Reboredo S. Chem-Eur. J. 2013, 19, 17244. |
| [20] | (b) Claraz A.; Masson G. Org. Biomol. Chem. 2018, 16, 5386. |
| [20] | (c) Yang B.; Wu X. X. Synthesis 2020, 53, 889. |
| [21] | (a) Mu?iz K. Acc. Chem. Res. 2018, 51, 1507. |
| [21] | (b) Jiang D. F.; Qi Z.; Li D.; Wen S. M.; Liu Z.; Hao W. J.; Jiang B. Org. Biomol. Chem. 2023, 21, 6757. |
| [21] | (c) Chen J.; Qu H. M.; Peng J.; Chen C. Chin. J. Org. Chem. 2015, 35, 937 (in Chinese). |
| [21] | (陈静, 曲红梅, 彭静, 陈超, 有机化学, 2015, 35, 937.) |
| [22] | Vemuri P. Y.; Patureau F. W. Org. Lett. 2021, 23, 3902. |
| [23] | (a) Kubota K.; Seo T.; Koide K.; Hasegawa Y.; Ito H. Nat. Commun. 2019, 10, 111. |
| [23] | (b) Kubota K.; Takahashi R.; Uesugi M.; Ito H. ACS Sustainable Chem. Eng. 2020, 8, 16577. |
| [23] | (c) Kubota K.; Endo T.; Uesugi M.; Hayashi Y.; Ito H. ChemSusChem 2022, 15, e202102132. |
| [23] | (d) Lemesre Q.; Wiesner T.; Wiechert R.; Rodrigo E.; Triebel S.; Geneste H. Green Chem. 2022, 24, 5502. |
/
| 〈 |
|
〉 |