

Preparation of Ni/Zn/Al Type Hydrotalcite Non-Noble Metal Catalysts and Application in the Synthesis of N-Substituted Indole Derivatives Via Alcoholamine Method
Received date: 2024-03-10
Online published: 2024-05-17
Supported by
Research on Key Technologies for Safe and Efficient Large scale Hydrogen Storage(2023ZZ1205); Sichuan Science and Technology Program(2021ZYCD005); Science and Technology Project of Southwest Petroleum University(2021JBGS07)
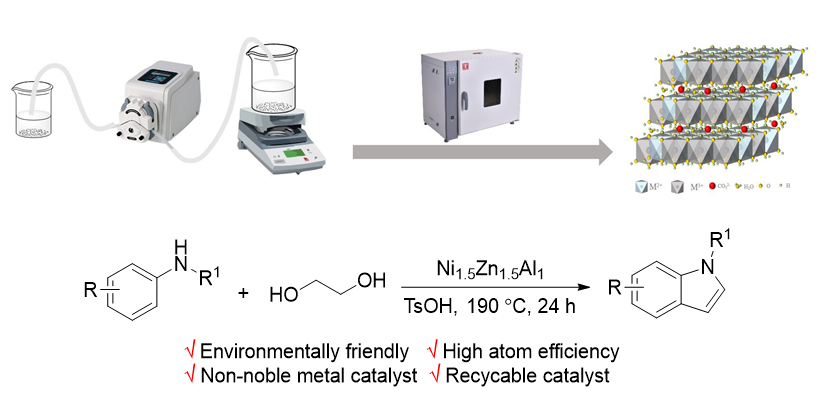
Indole derivatives are an extremely important class of heterocyclic compounds with extensive applications in fields such as medicine and biology. In recent years, with the development of organic liquid hydrogen storage technology, its application scope has been continuously expanding. There have been many reports on the synthesis of indole, among which the synthesis of monosubstituted indole mainly involves direct halogenation of indole and direct cyclization of aromatic amines and alcohols. The former requires a large amount of halogenated reagents and excessive inorganic salts, resulting in poor environmental compatibility. The latter is a very ideal synthesis method with advantages such as high atomic efficiency and environmental friendliness, but currently all reported cases require the participation of precious metals. Therefore, the development of inexpensive non precious metal catalysts based on biomass ethylene glycol as raw material for the synthesis of N-substituted indole compounds has important scientific research significance and application value in large-scale production and application in the fields of medicine and hydrogen energy. In the current study, a series of hydrotalcite like catalysts using the coprecipitation method were prepared and applied in the one-step synthesis of N-substituted indoles using ethylene glycol and N-substituted anilines. By changing the elemental composition of hydrotalcite like catalysts, the optimal elemental composition was selected as Ni1.5Zn1.5Al1, with Ni as the main active component playing a catalytic role, Zn element can synergistically promote the reaction with Ni element, while aluminum element is a structural maintenance component without catalytic activity. The structure and composition of Ni/Zn/Al hydrotalcite catalysts were analyzed by X-ray diffraction (XRD), thermogravimetric analysis (TGA), inductively coupled plasma-optical emission spectrometer (ICP-OES), and N2 physical adsorption analysis (BET), scanning electron microscopy (SEM), and transmission electron microscopy (TEM). This strategy can be effectively applied to N-alkyl and aryl indole derivatives. Compared with reported methods, it overcomes the limitations of precious metals, and the catalyst can maintain good catalytic activity after five cycles. It has the advantages of low raw material price, low production cost, simple operation process, friendly environment and high atomic utilization rate.
Lei Li , Junlei Tang , Litao Wang , Jiangtao Li , Honglin Quan , Bing Lin , Hong Wang , Jia-Qi Li , Taigang Zhou . Preparation of Ni/Zn/Al Type Hydrotalcite Non-Noble Metal Catalysts and Application in the Synthesis of N-Substituted Indole Derivatives Via Alcoholamine Method[J]. Acta Chimica Sinica, 2024 , 82(7) : 748 -754 . DOI: 10.6023/A24030077
| [1] | Thanikachalam P.-V.; Maurya R.-K.; Garg V.; Monga V. Eur. J. Med. Chem. 2019, 180, 562. |
| [2] | Mathada B.-S.; Yernale N.-G.; Basha J.-N. Chemistryselect 2023, 8, 1. |
| [3] | Kumar D.; Kumar N.-M.; Sundaree S.; Johnson E.-O.; Shah K. Eur. J. Med. Chem. 2010, 45, 1244. |
| [4] | Russo E.; Grondona C.; Brullo C.; Spallarossa A.; Villa C.; Tasso B. Pharmaceutics 2023, 15, 1815. |
| [5] | Han Y.; Dong W.; Guo Q. ; Li X.; Huang L. Eur. J. Med. Chem. 2020, 203, 112506. |
| [6] | Dadashpour S.; Emami S. Eur. J. Med. Chem. 2018, 150, 9. |
| [7] | Alharthi S.-S. Chem. Pap. 2023, 77, 7379. |
| [8] | Sun P.; Huang Y.; Yang X.; Liao A.; Wu J. Front. Plant Sci. 2023, 13, 1. |
| [9] | Kalepu J.; Gandeepan P.; Ackermann L.; Pilarski L.-T. Chem. Sci. 2018, 9, 4203. |
| [10] | Pang Q.; Zuo W.-F.; Zhang Y.; Li X.; Han B. Chem. Rec. 2023, 23, 2. |
| [11] | Li X. Y.; Tang J. L.; Li J. Q.; Lei X. Z.; Zhou T. G. Low-Carbon Chemistry and Chemical Engineering 2023, 6, 107 (in Chinese). |
| [11] | (李欣雨, 唐鋆磊, 李佳奇, 雷宪章, 周太刚, 低碳化学与化工, 2023, 6, 107.) |
| [12] | Yang M.; Cheng G.; Xie D.; Zhu T.; Dong Y.; Ke H.; Cheng H. Int. J. Hydrogen Energ. 2018, 43, 8868. |
| [13] | Dong Y.; Yang M.; Li L.; Zhu T.; Chen X.; Cheng H. Int. J. Hydrogen Energ. 2019, 44, 4919. |
| [14] | Dong Y.; Zhao H.; Zhao Y.; Yang M.; Zhang H.; Cheng H. RSC Adv. 2021, 11, 15729. |
| [15] | Lee J.; Park B.-G.; Sung K.; Lee H.; Kim J.; Nam E.; Han J. -W.; An K. ACS Catal. 2023, 13, 13691. |
| [16] | S?gaard A.; Scheuermeyer M.; B?smann A.; Wasserscheid P.; Riisager A. Chem. Commun. 2019, 55, 2046. |
| [17] | Cacchi S.; Fabrizi G. Chem. Rev. 2005, 105, 2873. |
| [18] | Humphrey G.-R.; Kuethe J.-T. Chem. Rev. 2006, 106, 2875. |
| [19] | Zheng L.; Tao K.; Guo W. Adv. Synth. Catal. 2021, 363, 62. |
| [20] | Neto J.-S.-S.; Zeni G. Asian J. Org. Chem. 2021, 10, 1282. |
| [21] | Tsuji Y.; Huh K.-T.; Watanabe Y. Tetrahedron Lett. 1986, 27, 377. |
| [22] | Zhang M.; Xie F.; Wang X.; Yan F.; Wang T.; Chen M.; Ding Y. RSC Adv. 2013, 3, 6022. |
| [23] | Minakawa M.; Watanabe K.; Toyoda S.; Uozumi Y. Synlett 2018, 29, 2385. |
| [24] | Chang H. H.; Zhang J.; Yan X. Y.; Li J. X.; Liu Q.; Gao W. C. CN 113773241, 2021 [Chem. Abstr. 2021, 177, 129151]. |
| [25] | Kumari S.; Sharma A.; Kumar S.; Thakur A.; Thakur R.; Bhatia S.-K.; Sharma A.-K. Chemosphere 2022, 306, 135464. |
| [26] | Trujillano R.; Labajos F.-M.; Rives V. Appl. Clay Sci. 2023, 238, 106927. |
| [27] | Baskaran T.; Christopher J.; Sakthivel A. RSC Adv. 2015, 5, 98853. |
| [28] | Cheng J.; Wang D. S. Chin. J. Cat. 2022, 43, 1380. |
| [29] | Li J. X.; Li B.; Wang J. K.; He L.; Zhao Y. F. Acta Chim. Sinica 2021, 79, 238 (in Chinese). |
| [29] | (李佳欣, 李蓓, 王纪康, 何蕾, 赵宇飞, 化学学报, 2021, 79, 238.) |
| [30] | Yu J.; Yang Y. S.; Wei M. Acta Chim. Sinica 2019, 77, 1129 (in Chinese). |
| [30] | (余俊, 杨宇森, 卫敏, 化学学报, 2019, 77, 1129.) |
| [31] | Gupta S.-S.-R.; Nakhate A.-V.; Rasal K.-B.; Deshmukh G.-P.; Mannepalli L.-K. New J. Chem. 2017, 41, 15268. |
| [32] | Sardar B.; Jamatia R.; Samanta A.; Srimani D. J. Org. Chem. 2022, 87, 5556. |
| [33] | Chatla A.; Abu-Rub F.; Prakash A.-V.; Ibrahim G.; Elbashir N.-O. Fuel 2022, 308, 122042. |
| [34] | Cantrell D.-G.; Gillie L.-J.; Lee A.-F.; Wilson K. Appl. Catal. A- Gen. 2005, 287, 183. |
| [35] | Smoláková L.; Frolich K.; Kocík J.; Kikhtyanin O. Ind. Eng. Chem. Res. 2017, 56, 4638. |
| [36] | Jiang W.; Lu H.; Qi T.; Yan S.; Liang B. Biotechnol. Adv. 2010, 28, 620. |
| [37] | Ouyang Y.; Xu Y.; Zhao L.; Deng M.; Yang P.; Peng G.; Ke G. Sci. Rep.-Uk. 2021, 11, 21625. |
/
| 〈 |
|
〉 |