

Designed Synthesis of SBA-15 Supported Sulfated Zirconia Solid Acid Materials and Their Catalytic Performance for Transesterification Reaction
Received date: 2024-03-21
Online published: 2024-05-17
Supported by
National Natural Science Foundation of China(21975174); National Natural Science Foundation of China(22378286)
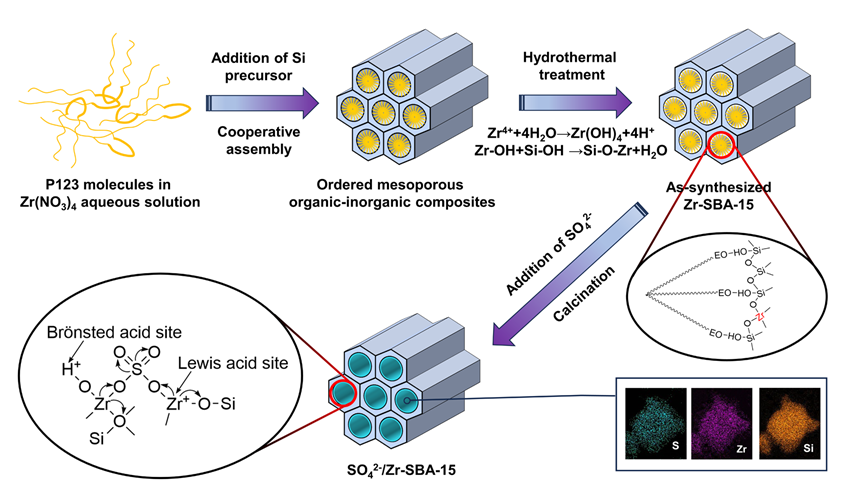
Ordered mesoporous Zr-SBA-15 material with a highly homogenous distribution of Zr within mesoporous walls was synthesized by a facile one-step high temperature hydrothermal treatment approach. In the proposed approach, the cooperative self-assembly between amphiphilic triblock copolymer P123 and silicon hydroxyl (Si-OH) species from the hydrolysis of tetraethyl orthosilicate (TEOS) was carried out to form an organic-inorganic composite with an ordered mesostructure (as-synthesized SBA-15, silica matrices with an embeddedness of P123 micelles) under a weak acidic medium originated from the hydrolysis of zirconium tetranitrate; during the followed high temperature hydrothermal treatment process, the further hydrolysis of zirconium precursor promotes more Zr4+ transferring to zirconium hydroxyl (Zr-OH) species, and the condensation between the Zr-OH species and Si-OH species, which exist in the synthesis solution and within the mesoporous walls of as-synthesized SBA-15, respectively, leads to a large amount of Zr atoms being highly homogenously grafted into the mesoporous walls of as-synthesized SBA-15 and the formation of framework Zr—O—Si bonds. After calcination to remove the organic P123 micelles, the ordered mesoporous Zr-SBA-15 material was obtained and used as a support for the immobilization of sulfuric acid by a traditional impregnation method. The characterization results confirmed that compared to supports SBA-15 and SBA-15-supported zirconia (Zr/SBA-15, prepared by the impregnation of SBA-15 with zirconium tetranitrate aqueous soliton followed drying and calcination treatments), the use of support Zr-SBA-15 can realize a stable immobilization of much more sulfuric acid groups, due to the highly homogenous dispersions of ZrOx species on the mesoporous surface of Zr-SBA-15. In addition, the double attracting electron role from supported sulfuric acid group and Si element with higher electronegativity than that of Zr element can further induce an decrease in the electron cloud density of Zr4+ existing in the surface Zr—O—Si bonds, which plays an important role in promoting the formation of super strong Brönsted and Lewis acidic sites. More importantly, the formation of surface Zr—O—Si bonds can exert a significant influence on restraining the leaching of active sulfated ZrOx species during the reaction. The resultant solid acid SO42−/Zr-SBA-15 exhibits an ordered two-dimensional hexagonal mesostructure, a uniform mesoporous size, a high specific surface area, a large pore volume, and a prominently improved acidity. For soybean oil transesterification with methanol, the catalyst SO42−/Zr-SBA-15 demonstrated a much higher soybean oil conversion (up to 100%) along with a fatty acid methyl ester yield of 99.8% and a desirable reusability, compared to the traditional supported sulfated zirconia catalysts. The superior catalytic performance of SO42−/Zr-SBA-15 is associated with the outstanding structural and textural properties of support Zr-SBA-15 and the for-mation of surface Zr—O—Si bonds, thus benefiting the fast mass and heat transfer and the generation of a large number of stable active sites with a highly homogenous dispersion.
Key words: mesoporous material; solid acids; sulfated zirconia; Zr-SBA-15; transesterification
Xiaoxuan Duan , Hengyan Wang , Dahai Pan , Shuwei Chen , Feng Yu , Xiaoliang Yan , Ruifeng Li . Designed Synthesis of SBA-15 Supported Sulfated Zirconia Solid Acid Materials and Their Catalytic Performance for Transesterification Reaction[J]. Acta Chimica Sinica, 2024 , 82(7) : 755 -762 . DOI: 10.6023/A24030095
| [1] | Tang W.-Q.; Gao M.; Zhang B.-X.; Wang X.-N.; Wu C.-F.; Wang Q.-H.; Liu S. J. Environ. Chem. Eng. 2023, 11, 109797. |
| [2] | Jiang L.; Fan Y.-Q.; Zhang X.-X.; Pei Y.; Yan S.-R.; Qiao M.-H.; Fan K.-N.; Zong B.-N. Acta Chim. Sinica 2023, 81, 231 (in Chinese). |
| [2] | (姜兰, 范义秋, 张晓昕, 裴燕, 闫世润, 乔明华, 范康年, 宗保宁, 化学学报, 2023, 81, 231.) |
| [3] | Fatimah I.; Yanti I.; Suharto T. E.; Sagadevan S. Inorg. Chem. Commun. 2022, 143, 109808. |
| [4] | Parekh A.; Mishra M. K.; Shukla A. D. Appl Catal, A. 2023, 663, 119268. |
| [5] | Rattanaphra D.; Temrak A.; Nuchdang S.; Kingkam W.; Puripunyavanich V.; Thanapimmetha A.; Saisriyoot M.; Srinophakun P. Energy Rep. 2021, 7, 5374. |
| [6] | Wang G.; Li Z.-X. Chem. Eng. J. 2022, 432, 134395. |
| [7] | Wang S.; Meng X.; Liu N.-W.; Shi L. Sep. Purif. Technol. 2023, 308, 122731. |
| [8] | Sekewael S. J.; Pratika R. A.; Hauli L.; Amin A. K.; Utami M.; Wijaya K. Catalysts 2022, 12, 191. |
| [9] | Delarmelina M.; Deshmukh G.; Goguet A.; Catlow C. R. A.; Manyar H. J. Phys. Chem. C 2021, 125, 27578. |
| [10] | Wang S.; Pu J.-L.; Wu J.-Q.; Liu H.-J.; Xu H.-Y.; Li X.; Wang H. ACS Omega 2020, 5, 30139. |
| [11] | Wang P.-Z.; Yue Y.-Y.; Wang T.-H.; Bao X.-J. Int. J. Energy Res. 2020, 44, 3270. |
| [12] | Colmenares-Zerpa J.; Gajardo J.; Peixoto A. F.; Silva D. S. A.; Silva J. A.; Gispert-Guirado F.; Llorca J.; Urquieta-Gonzalez E. A.; Santos J. B. O.; Chiment?o R. J. J. Solid. State. Chem. 2022, 312, 123296. |
| [13] | Liu Z.-H.; Zhang Z.-Z.; Zhou Y.-L.; Wang Z.-L.; Du M.-Y.; Wen Z.; Yan B.; Ma Q.-X.; Liu N.; Xue B. Fuel 2024, 356, 129631. |
| [14] | Colmenares-Zerpa J.; Chiment?o R. J.; Gispert-Guirado F.; Peixoto A. F.; Llorca J. Mater. Lett. 2021, 301, 130326. |
| [15] | Jin D.-F.; Hu G.-B.; Jin H.-X.; Wang X.-Q.; Hong B.; Peng X.-L.; Ge H.-L. Rare Metal Mat. Eng. 2012, 41, 379. |
| [16] | Huo L.-M.; Wang T.; Xuan K.; Li L.; Pu Y.-F.; Li C.-X.; Qiao C.-Z.; Yang H.; Bai Y. Catalysts 2021, 11, 710. |
| [17] | Tang Y.-Q.; Zong E.-M.; Wan H.-Q.; Xu Z.-Y.; Zheng S.-R.; Zhu D.-Q. Microporous Mesoporous Mater. 2012, 155, 192. |
| [18] | Sabbaghi A.; Lam F. L. Y.; Hu X.-J. J. Mol. Catal. A: Chem. 2015, 409, 69. |
| [19] | Ahn H.; Chen H. W.; Landheer D.; Wu X.; Chou L. J.; Chao T. S. Thin Solid Films 2004, 455-456, 318. |
| [20] | Sabbaghi A.; Lam F. L. Y.; Hu X.-J. Appl. Catal., A 2015, 508, 25. |
| [21] | Chen S.-Y.; Lee J.-F.; Cheng S. J. Catal. 2010, 270, 196. |
| [22] | Thunyaratchatanon C.; Luengnaruemitchai A.; Chaisuwan T.; Chollacoop N.; Chen S.-Y.; Yoshimura Y. Microporous Mesoporous Mater. 2017, 253, 18. |
| [23] | Wei Y.-F.; Li Y.-F.; Tan Y.; Zhou J.; Wu Z.-M.; Liu Y.-J. Mater. Lett. 2015, 141, 145. |
| [24] | Sareen S.; Mutreja V.; Singh S.; Pal B. J. Colloid Interface Sci. 2016, 461, 203. |
| [25] | Gajardo J.; Colmenares-Zerpa J.; Peixoto A. F.; Silva D. S. A.; Silva J. A.; Gispert-Guirado F.; Llorca J.; Urquieta-Gonzalez E. A.; Santos J. B. O.; Szanyi J.; Sepúlveda C.; álvarez M. G.; Chiment?o R. J. J. Porous Mater. 2023, 30, 1687. |
| [26] | Krishnan C. K.; Hayashi T.; Ogura M. Adv. Mater. 2008, 20, 2131. |
| [27] | Liu X.-X.; Wang K.; Liu B.-Q.; Guo Z.-M.; Zhang C.; Lv Z.-G. J. Solid State Chem. 2021, 300, 122239. |
| [28] | Naghavi M.; Mazloom G.; Akbari A.; Banisharif F. Chem. Eng. Res. Des. 2021, 174, 454. |
| [29] | Ren K.-Q.; Kong D.-C.; Meng X.; Wang X.; Shi L.; Liu N.-W. J. Saudi Chem. Soc. 2019, 23, 198. |
| [30] | Ganiyu S. A.; Alhooshani K. Energy Fuels 2019, 33, 3047. |
| [31] | Noda L. K.; De Almeida R. M.; Probst L. F. D.; Gon?alves N. S. J. Mol. Catal. A: Chem. 2005, 225, 39. |
| [32] | Yan G. X.; Wang A.-Q.; Wachs I. E.; Baltrusaitis J. Appl. Catal., A 2019, 572, 210. |
| [33] | Rizwanul Fattah I. M.; Ong H. C.; Mahlia T. M. I.; Mofijur M.; Silitonga A. S.; Rahman S. M. A.; Ahmad A. Front. Energy Res. 2020, 8, 101. |
| [34] | Garcia C.; Teixeira S.; Marciniuk L.; Schuchardt U. Bioresour. Technol. 2008, 99, 6608. |
/
| 〈 |
|
〉 |