

Metal Ion-gemcitabine Monophosphate Nanoparticles for Effective Treatment of Pancreatic Cancer
Received date: 2024-03-14
Online published: 2024-06-04
Supported by
Chongqing Doctoral Research Project(CSTB2022BSXM-JCX0127); Innovation Leading Talents of Chongqing Talent Program(425Z2P12D)
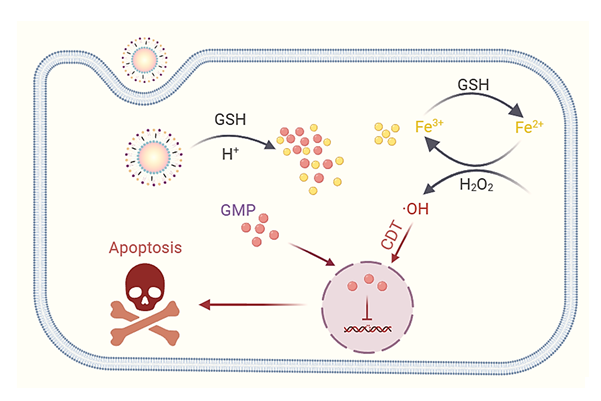
Gemcitabine is the first-line chemotherapeutic agent and the gold standard for pancreatic cancer. However, the short blood half-life and drug resistance limit its therapeutic efficacy in clinic. Recent advancements in nanotechnology offered a potent approach for improving gemcitabine delivery and efficacy. In this study, we present the design and synthesis of gemcitabine-loaded nanoparticles utilizing gemcitabine monophosphate (GMP) and metal ions via a coordination-precipitation strategy. Firstly, a series of metal ions, including Ca2+, Fe3+, Mn2+, Gd3+ and Lu3+, were mixed with GMP to form nanoparticles (Metal-GMP NPs) employing a reverse-phase microemulsion method. The morphology of the as-prepared Metal-GMP NPs were characterized by transmission electron microscopy while the hydrated particle size, polydispersion index and zeta potential were measured by dynamic light scattering. Additionally, the encapsulation efficiency and loading ratio of GMP, as well as the molar ratio of GMP to metals were quantified through high performance liquid chromatography (HPLC) and inductively coupled plasma mass spectrometry (ICP-MS). Subsequently, the anti-tumor efficacy of the as-prepared Metal-GMP NPs were studied by CCK8 assay on three different pancreatic cancer cell lines. The screening results suggested that Fe-GMP NPs has the preferable anti-tumor activity as well as the obvious synergistic effect between metal ions and drugs. The further mechanism studies revealed that Fe-GMP NPs could generate enough cytotoxic reactive oxygen species (ROS) through Fenton-like reactions within tumor cells. And simultaneously, the intracellular reduced glutathione (GSH) was consumed, thereby disrupting the redox homeostasis and enhancing GEM efficacy. Finally, the therapeutic efficacy and bio-safety of Fe-GMP NPs were investigated on the subcutaneous tumor-bearing model. The in vivo results demonstrated that Fe-GMP NPs exhibited better therapeutic efficacy rather than GMP free drugs alone. Meanwhile, the bio-safety assessment confirmed the absence of significant toxicity or side effects associated with Fe-GMP NP treatment. This work combines metal ions with gemcitabine in the manner of chemistry, providing a highly promising strategy for the delivery and sensitization of gemcitabine in pancreatic cancer therapy.
Qianyu Luo , Chengyan Wang , Tianlong Zhang , Peiyuan Xia , Xiao Zhang , Ming Yang . Metal Ion-gemcitabine Monophosphate Nanoparticles for Effective Treatment of Pancreatic Cancer[J]. Acta Chimica Sinica, 2024 , 82(7) : 772 -781 . DOI: 10.6023/A24030085
| [1] | Siegel R. L.; Miller K. D.; Wagle N. S.; Jemal A. Ca-Cancer J. Clin. 2023, 73, 17. |
| [2] | Strobel O.; Neoptolemos J.; J?ger D.; Büchler M. W. Nat. Rev. Clin. Oncol. 2018, 16, 11. |
| [3] | Saung M. T.; Zheng L. Clin. Ther. 2017, 39, 2125. |
| [4] | Gu Z. Y.; Du Y. X.; Zhao X.; Wang C. Cancer Lett. 2021, 521, 98. |
| [5] | Dubey R. D.; Saneja A.; Gupta P. K.; Gupta P. N. Eur. J. Pharm. Sci. 2016, 93, 147. |
| [6] | Saiki Y.; Yoshino Y.; Fujimura H.; Manabe T.; Kudo Y.; Shimada M.; Mano N.; Nakano T.; Lee Y.; Shimizu S.; Oba S.; Fujiwara S.; Shimizu H.; Chen N.; Nezhad Z. K.; Jin G.; Fukushige S.; Sunamura M.; Ishida M.; Motoi F.; Egawa S.; Unno M.; Horii A. Biochem. Biophys. Res. Commun. 2012, 421, 98. |
| [7] | Yamamoto M.; Sanomachi T.; Suzuki S.; Uchida H.; Yonezawa H.; Higa N.; Takajo T.; Yamada Y.; Sugai A.; Togashi K.; Seino S.; Okada M.; Sonoda Y.; Hirano H.; Yoshimoto K.; Kitanaka C. Neuro-oncol. 2021, 23, 945. |
| [8] | Li X.; Porcel E.; Menendez‐Miranda M.; Qiu J. W.; Yang X. M.; Serre C.; Pastor A.; Desma?le D.; Lacombe S.; Gref R. ChemMedChem 2019, 15, 274. |
| [9] | Das M.; Li J.; Bao M.; Huang L. AAPS J. 2020, 22, 88. |
| [10] | Sun H.; Cai H.; Xu C.; Zhai H. Z.; Lux F.; Xie Y.; Feng L.; Du L. Q.; Liu Y.; Sun X. H.; Wang Q.; Song H. J.; He N. N.; Zhang M. M.; Ji K. H.; Wang J. J.; Gu Y. Q.; Leduc G.; Doussineau T.; Wang Y.; Liu Q.; Tillement O. J. Nanobiotechnol. 2022, 20, 449. |
| [11] | Chen Y.; Huang Y. K.; Zhou S. L.; Sun M. L.; Chen L.; Wang J. H.; Xu M. J.; Liu S. S.; Liang K. F.; Zhang Q.; Jiang T. Z.; Song Q. X.; Jiang G.; Tang X. J.; Gao X. L.; Chen J. Nano Lett. 2020, 20, 6780. |
| [12] | Lv Y. G.; Chen X.; Shen Y. P. Carbohydr. Polym. 2024, 323, 121434. |
| [13] | Cai Z.; Zhang Y. W.; Jiang L. P.; Zhu J. J. Acta Chim. Sinica 2021, 79, 481 (in Chinese). |
| [13] | (蔡政, 张颖雯, 姜立萍, 朱俊杰, 化学学报, 2021, 79, 481.) |
| [14] | Li J. J.; Wei J. J.; Gao Y. X.; Zhao Q.; Sun J. J.; Ouyang J.; Na N. Chin. Chem. Lett. 2023, 34, 107662. |
| [15] | Cordani M.; Resines-Urien E.; Gamonal A.; Milán-Rois P.; Salmon L.; Bousseksou A.; Costa J. S.; Somoza á. Antioxidants 2021, 10, 66. |
| [16] | Guo W.; Hu C. Y.; Zhen S. J.; Huang C. Z.; Li Y. F. Acta Chim. Sinica 2022, 80, 1583 (in Chinese). |
| [16] | (郭湾, 胡聪意, 甄淑君, 黄承志, 李原芳, 化学学报, 2022, 80, 1583.) |
| [17] | Yu X. Y.; Shang T. Y.; Zheng G. D.; Yang H. L.; Li Y. W.; Cai Y. J.; Xie G. X.; Yang B. Chin. Chem. Lett. 2022, 33, 1895. |
| [18] | Simón M.; J?rgensen J. T.; Khare H. A.; Christensen C.; Nielsen C. H.; Kjaer A. Pharmaceutics 2022, 14, 1284. |
| [19] | Wang J.; Zhuo L. G.; Zhao P.; Liao W.; Wei H. Y.; Yang Y. C.; Peng S. M.; Yang X. Chin. Chem. Lett. 2022, 33, 3502. |
| [20] | Man X. Y.; Yang T. F.; Li W. J.; Li S. H.; Xu G.; Zhang Z. L.; Liang H.; Yang F. J. Med. Chem. 2023, 66, 7268. |
| [21] | Sun H. S.; Zhou J.; Liu C.; Chen X.; Du Y. J.; Li Y. L.; Jiang H.; Wang J. Q.; Song Z.; Guo C. Acta Chim. Sinica 2022, 80, 1250 (in Chinese). |
| [21] | (孙宏顺, 周进, 刘成, 陈旭, 杜怡璟, 李玉龙, 蒋蕻, 王建强, 宋喆, 郭成, 化学学报, 2022, 80, 1250.) |
| [22] | Chou T. C. Cancer Res. 2010, 70, 440. |
| [23] | Yang Z. B.; Luo Y.; Hu Y. N.; Liang K. C.; He G.; Chen Q.; Wang Q. G.; Chen H. Adv. Funct. Mater. 2020, 31, 2007991. |
| [24] | Bai Y.; Pan Y. J.; An N.; Zhang H. T.; Wang C.; Tian W.; Huang T. Chin. Chem. Lett. 2023, 34, 107552. |
| [25] | Dong L. P.; Ding J. S.; Zhu L. M.; Liu Y. J.; Gao X.; Zhou W. H. Chin. Chem. Lett. 2023, 34, 108192. |
| [26] | Yang Y. H.; Wang Y. F.; Xu L. G.; Chen T. Chin. Chem. Lett. 2020, 31, 1801. |
| [27] | Raza A.; Hayat U.; Rasheed T.; Bilal M.; Iqbal H. M. N. Eur. J. Med. Chem. 2018, 157, 705. |
| [28] | Ouyang J.; Wang L. Q.; Chen W.; Zeng K.; Han Y.; Xu Y.; Xu Q.; Deng L.; Liu Y.-N. Chem. Commun. 2018, 54, 3468. |
| [29] | Lei G.; Zhuang L.; Gan B. Nat. Rev. Cancer. 2022, 22, 381. |
/
| 〈 |
|
〉 |