

Ring-opening Copolymerization of Epoxides and Anhydride Mediated by Claw-type Aminophenolate Zinc Chlorides
Received date: 2024-04-08
Online published: 2024-06-04
Supported by
National Natural Science Foundation of China(21871082)
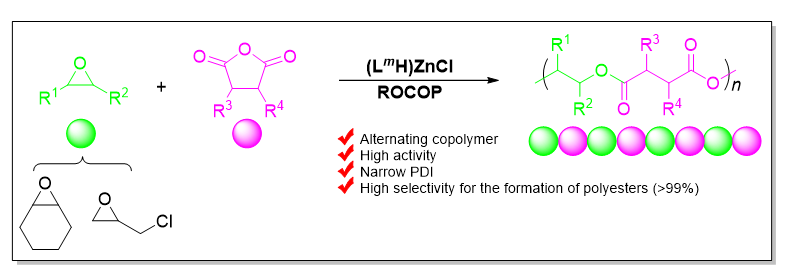
A series of novel claw-type aminophenolate zinc chlorides Zn1~Zn8 were synthesized via the reactions of the corresponding sodium salts of the proligands H1-8L with one equiv. of zinc dichloride at room temperature respectively. All the complexes were characterized by 1H NMR, 13C NMR spectroscopy and elemental analyses. The X-ray diffraction analysis of complex Zn2 showed that, being obtained as a pair of racemic isomers (with configurations of SNSZn and RNRZn), the complex possesses a mononuclear structure and the zinc center is four-coordinated by the tridentate aminophenolate ligand and one chloride ligand in the solid state. When bis(triphenylphosphine)iminium chloride (PPNCl) was used as a co-catalyst, all of these zinc complexes showed high catalytic activities and high selectivities for the formation of polyesters (>99%) in the ring-opening copolymerization (ROCOP) of cyclohexene oxide (CHO) and phthalic anhydride (PA), giving alternative copolymers with narrow molecular weight distributions (PDI=1.14~1.24). The steric hindrance of the substituents on the ligand has an important influence on the catalytic activity of the complex. Zn4 with cumyl groups on the ligand that make the metal center more crowded showed a lower activity than Zn3 at 80 ℃ (Zn3, TOFPA=400 h-1, Zn4, TOFPA=384 h-1). Moreover, the electron-withdrawing effect of the substituents increased the catalytic activity of the catalyst. For example, under the same conditions, Zn1 with electron-withdrawing substituents took only 22 min to achieve 90% conversion of PA, while Zn2 took 26 min to achieve 87% conversion of PA. Complex Zn1 showed the highest catalytic activity in the copolymerization of CHO and PA (TOF=490 h-1). In addition, with the increase of the amount of PPNCl, the selectivity of complexes for ester linkages gradually increased. For instance, when one equiv. of PPNCl was added, the selectivity of Zn2 for ester linkages was 96%, while a selectivity of 99% was achieved with the addition of 5 equiv. of PPNCl. All the complexes were also applied in the ROCOP of rac-epichlorohydrin (rac-ECH) and PA, and showed high catalytic activities and high selectivities for polyesters (>99%), but with somewhat wide molecular weight distributions (PDI=1.36~1.49). Complex Zn1 also proved to be the most active one (TOF=848 h-1). Compared with those for CHO and PA copolymerization, the catalytic activities of the complexes toward the ROCOP of rac-ECH and PA were higher (e.g. Zn2, TOF=402 h-1, CHO/PA; TOF=745 h-1, ECH/PA). Further studies suggested that the ROCOP was likely initiated through the ring-opening of epoxides upon the attack of an nucleophilic reagent, for instance, Cl-.
Haicheng Wang , Haiyan Ma . Ring-opening Copolymerization of Epoxides and Anhydride Mediated by Claw-type Aminophenolate Zinc Chlorides[J]. Acta Chimica Sinica, 2024 , 82(6) : 577 -588 . DOI: 10.6023/A24040122
| [1] | Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Chem. Rev. 2004, 104, 6147. |
| [2] | Williams, C. K. Chem. Soc. Rev. 2007, 36, 1573. |
| [3] | Williams, C. K.; Hillmyer, M. Polym. Rev. 2008, 48, 1. |
| [4] | Vroman, I.; Tighzert, L. Materials 2009, 2, 307. |
| [5] | Thomas, C. M. Chem. Soc. Rev. 2010, 39, 165. |
| [6] | Vilela, C.; Sousa, A. F.; Fonseca, A. C.; Serra, A. C.; Coelho, J. F. J.; Freire, C. S. R.; Silvestre, A. J. D. Polym. Chem. 2014, 5, 3119. |
| [7] | Zhou, Z.; Liu, S.; Lang, T.; Gao, F.; Wang, X. Acta Polym. Sin. 2024, 55, 396. (in Chinese) |
| [7] | (周振震, 刘顺杰, 郎涛涛, 高凤翔, 王献红, 高分子学报, 2024, 55, 396.) |
| [8] | Sanford, M. J.; Pe?a Carrodeguas, L.; Van Zee, N. J.; Kleij, A. W.; Coates, G. W. Macromolecules 2016, 49, 6394. |
| [9] | Hu, L.-F.; Zhang, C.-J.; Wu, H.-L.; Yang, J.-L.; Liu, B.; Duan, H.-Y.; Zhang, X.-H. Macromolecules 2018, 51, 3126. |
| [10] | Ji, H.-Y.; Wang, B.; Pan, L.; Li, Y.-S. Angew. Chem. Int. Ed. 2018, 57, 16888. |
| [11] | Liu, Y.; Guo, J.-Z.; Lu, H.-W.; Wang, H.-B.; Lu, X.-B. Macromolecules 2018, 51, 771. |
| [12] | St??er, T.; Williams, C. K. Angew. Chem. Int. Ed. 2018, 57, 6337. |
| [13] | Liu, F.-P.; Li, J.; Liu, Y.; Ren, W.-M.; Lu, X.-B. Macromolecules 2019, 52, 5652. |
| [14] | Zhou, Y.; Hu, C.; Zhang, T.; Xu, X.; Duan, R.; Luo, Y.; Sun, Z.; Pang, X.; Chen, X. Macromolecules 2019, 52, 3462. |
| [15] | Chen, X.; Chen, G.; Tao, Y.; Wang, Y.; Lu, X.; Zhang, L.; Zhu, J.; Zhang, J.; Wang, X. Acta Polym. Sin. 2019, 50, 1068. (in Chinese) |
| [15] | (陈学思, 陈国强, 陶友华, 王玉忠, 吕小兵, 张立群, 朱锦, 张军, 王献红, 高分子学报, 2019, 50, 1068.) |
| [16] | Lidston, C. A. L.; Abel, B. A.; Coates, G. W. J. Am. Chem. Soc. 2020, 142, 20161. |
| [17] | Fang, J.; Hu, F. Chin. J. Catal. 2002, 23, 88. (in Chinese) |
| [17] | (房江华, 胡富陶, 催化学报, 2002, 23, 88.) |
| [18] | Huang, Y.; Qi, G.; Feng, L. Chin. J. Catal. 2002, 23, 113. (in Chinese) |
| [18] | (黄亦军, 戚国荣, 封麟先, 催化学报, 2002, 23, 113.) |
| [19] | Hua, Z.; Chen, S.; Fang, Z.; Qi, G. Acta Polym. Sin. 2004, 4, 551. (in Chinese) |
| [19] | (华正江, 陈上, 方佐, 戚国荣, 高分子学报, 2004, 4, 551.) |
| [20] | Huijser, S.; HosseiniNejad, E.; Sablong, R.; de Jong, C.; Koning, C. E.; Duchateau, R. Macromolecules 2011, 44, 1132. |
| [21] | Longo, J. M.; DiCiccio, A. M.; Coates, G. W. J. Am. Chem. Soc. 2014, 136, 15897. |
| [22] | Van Zee, N. J.; Coates, G. W. Chem. Commun. 2014, 50, 6322. |
| [23] | Baumgartner, R.; Song, Z.; Zhang, Y.; Cheng, J. Polym. Chem. 2015, 6, 3586. |
| [24] | Si, G.; Zhang, L.; Han, B.; Duan, Z.; Li, B.; Dong, J.; Li, X.; Liu, B. Polym. Chem. 2015, 6, 6372. |
| [25] | Zhu, Y.; Romain, C.; Poirier, V.; Williams, C. K. Macromolecules 2015, 48, 2407. |
| [26] | DiCiccio, A. M.; Longo, J. M.; Rodríguez-Calero, G. G.; Coates, G. W. J. Am. Chem. Soc. 2016, 138, 7107. |
| [27] | Han, B.; Zhang, L.; Yang, M.; Liu, B.; Dong, X.; Theato, P. Macromolecules 2016, 49, 6232. |
| [28] | Fieser, M. E.; Sanford, M. J.; Mitchell, L. A.; Dunbar, C. R.; Mandal, M.; Van Zee, N. J.; Urness, D. M.; Cramer, C. J.; Coates, G. W.; Tolman, W. B. J. Am. Chem. Soc. 2017, 139, 15222. |
| [29] | Zhou, Y.; Duan, R.; Li, X.; Pang, X.; Wang, X.; Chen, X. Chem. -Asian J. 2017, 12, 3135. |
| [30] | Bester, K.; Bukowska, A.; My?liwiec, B.; Hus, K.; Tomczyk, D.; Urbaniak, P.; Bukowski, W. Polym. Chem. 2018, 9, 2147. |
| [31] | Chen, T. T. D.; Zhu, Y.; Williams, C. K. Macromolecules 2018, 51, 5346. |
| [32] | Hiranoi, Y.; Nakano, K. Beilstein J. Org. Chem. 2018, 14, 2779. |
| [33] | Martínez, J.; Martínez de Sarasa Buchaca, M.; de la Cruz-Martínez, F.; Alonso-Moreno, C.; Sánchez-Barba, L. F.; Fernandez-Baeza, J.; Rodríguez, A. M.; Rodríguez-Diéguez, A.; Castro-Osma, J. A.; Otero, A.; Lara-Sánchez, A. Dalton Trans. 2018, 47, 7471. |
| [34] | Sanford, M. J.; Van Zee, N. J.; Coates, G. W. Chem. Sci. 2018, 9, 134. |
| [35] | Ji, H.-Y.; Song, D.-P.; Wang, B.; Pan, L.; Li, Y.-S. Green Chem. 2019, 21, 6123. |
| [36] | Kernbichl, S.; Reiter, M.; Mock, J.; Rieger, B. Macromolecules 2019, 52, 8476. |
| [37] | Li, J.; Liu, Y.; Ren, W.-M.; Lu, X.-B. Proc. Natl. Acad. Sci. 2020, 117, 15429. |
| [38] | Lin, L.; Liang, J.; Xu, Y.; Wang, S.; Xiao, M.; Sun, L.; Meng, Y. Green Chem. 2019, 21, 2469. |
| [39] | Li, J.; Ren, B.-H.; Chen, S.-Y.; He, G.-H.; Liu, Y.; Ren, W.-M.; Zhou, H.; Lu, X.-B. ACS Catal. 2019, 9, 1915. |
| [40] | Li, W.-B.; Liu, Y.; Lu, X.-B. Organometallics 2020, 39, 1628. |
| [41] | Ryu, H. K.; Bae, D. Y.; Lim, H.; Lee, E.; Son, K.-S. Polym. Chem. 2020, 11, 3756. |
| [42] | Shi, D.; Li, L.; Wen, Y.; Yang, Q.; Duan, Z. Polym. Int. 2020, 69, 513. |
| [43] | Sulley, G. S.; Gregory, G. L.; Chen, T. T. D.; Pe?a Carrodeguas, L.; Trott, G.; Santmarti, A.; Lee, K.-Y.; Terrill, N. J.; Williams, C. K. J. Am. Chem. Soc. 2020, 142, 4367. |
| [44] | Wang, L.; Zhang, J.; Zhao, N.; Ren, C.; Liu, S.; Li, Z. ACS Macro Lett. 2020, 9, 1398. |
| [45] | Zhu, S.; Wang, Y.; Ding, W.; Zhou, X.; Liao, Y.; Xie, X. Polym. Chem. 2020, 11, 1691. |
| [46] | Li, S.; Wang, Y.; Ji, H.; Chen, C.; Chen, X.; Pan, L.; Wang, B. Acta Polym. Sin. 2020, 51, 1039. (in Chinese) |
| [46] | (李帅, 王昱博, 季鹤源, 陈崇民, 陈晓璐, 潘莉, 王彬, 高分子学报, 2020, 51, 1039.) |
| [47] | Cui, L.; Ren, B.-H.; Lu, X.-B. J. Polym. Sci. 2021, 59, 1821. |
| [48] | Diment, W. T.; St??er, T.; Kerr, R. W. F.; Phanopoulos, A.; Durr, C. B.; Williams, C. K. Catal. Sci. Technol. 2021, 11, 1737. |
| [49] | Aida, T.; Inoue, S. J. Am. Chem. Soc. 1985, 107, 1358. |
| [50] | Jeon, J. Y.; Eo, S. C.; Varghese, J. K.; Lee, B. Y. Beilstein J. Org. Chem. 2014, 10, 1787. |
| [51] | Diment, W. T.; Gregory, G. L.; Kerr, R. W. F.; Phanopoulos, A.; Buchard, A.; Williams, C. K. ACS Catal. 2021, 11, 12532. |
| [52] | Liu, D.-F.; Wu, L.-Y.; Feng, W.-X.; Zhang, X.-M.; Wu, J.; Zhu, L.-Q.; Fan, D.-D.; Lü, X.-Q.; Shi, Q. J. Mol. Catal. A-Chem. 2014, 382, 136. |
| [53] | Saini, P. K.; Romain, C.; Zhu, Y.; Williams, C. K. Polym. Chem. 2014, 5, 6068. |
| [54] | Thevenon, A.; Garden, J. A.; White, A. J. P.; Williams, C. K. Inorg. Chem. 2015, 54, 11906. |
| [55] | Ji, H.-Y.; Wang, B.; Pan, L.; Li, Y.-S. Green Chem. 2018, 20, 641. |
| [56] | Wang, L.; Ma, H. Dalton Trans. 2010, 39, 7897. |
| [57] | Darensbourg, D. J.; Poland, R. R.; Escobedo, C. Macromolecules 2012, 45, 2242. |
| [58] | Li, J.; Liu, Y.; Ren, W.-M.; Lu, X.-B. J. Am. Chem. Soc. 2016, 138, 11493. |
| [59] | St??er, T.; Mulryan, D.; Williams, C. K. Angew. Chem. Int. Ed. 2018, 57, 16893. |
| [60] | Abel, B. A.; Lidston, C. A. L.; Coates, G. W. J. Am. Chem. Soc. 2019, 141, 12760. |
| [61] | Ryu, H. K.; Cha, J.; Yu, N.; Lee, E.; Son, K. S. Inorg. Chem. Commun. 2020, 122, 108278. |
| [62] | Wang, H.; Ma, H. Chem. Commun. 2013, 49, 8686. |
| [63] | Mundil, R.; Ho?t’álek, Z.; ?eděnková, I.; Merna, J. Macromol. Res. 2015, 23, 161. |
| [64] | Martínez de Sarasa Buchaca, M.; de la Cruz-Martínez, F.; Martínez, J.; Alonso-Moreno, C.; Fernández-Baeza, J.; Tejeda, J.; Niza, E.; Castro-Osma, J. A.; Otero, A.; Lara-Sánchez, A. ACS Omega 2018, 3, 17581. |
| [65] | Chen, C.-M.; Xu, X.; Ji, H.-Y.; Wang, B.; Pan, L.; Luo, Y.; Li, Y.-S. Macromolecules 2021, 54, 713. |
| [66] | Martínez, G.; Pedrosa, S.; Tabernero, V.; Mosquera, M. E. G.; Cuenca, T. Organometallics 2008, 27, 2300. |
| [67] | Ling, J.; You, L.; Wang, Y.; Shen, Z. J. Appl. Polym. Sci. 2012, 124, 2537. |
| [68] | Ambrose, K.; Murphy, J. N.; Kozak, C. M. Macromolecules 2019, 52, 7403. |
| [69] | Chen, P.; Chisholm, M. H.; Gallucci, J. C.; Zhang, X.; Zhou, Z. Inorg. Chem. 2005, 44, 2588. |
| [70] | Chisholm, M. H.; Navarro-Llobet, D.; Simonsick, W. J. Macromolecules 2001, 34, 8851. |
| [71] | Ho??álek, Z.; Trhlíková, O.; Walterová, Z.; Martinez, T.; Peruch, F.; Cramail, H.; Merna, J. Eur. Polym. J. 2017, 88, 433. |
| [72] | Xie, R.; Zhang, Y.-Y.; Yang, G.-W.; Zhu, X.-F.; Li, B.; Wu, G.-P. Angew. Chem. Int. Ed. 2021, 60, 19253. |
| [73] | Li, J.; Ren, B.-H.; Wan, Z.-Q.; Chen, S.-Y.; Liu, Y.; Ren, W.-M.; Lu, X.-B. J. Am. Chem. Soc. 2019, 141, 8937. |
| [74] | Huang, M.; Pan, C.; Ma, H. Dalton Trans. 2015, 44, 12420. |
| [75] | Yang, Y.; Wang, H.; Ma, H. Inorg. Chem. 2015, 54, 5839. |
/
| 〈 |
|
〉 |