

Design, Synthesis and Properties of Cu(I) Complexes with a Nitrogen-containing Spirocycle Ligand for Delayed Fluorescence Materials
Received date: 2024-04-30
Online published: 2024-06-13
Supported by
National Natural Science Foundation of China(52073286); National Natural Science Foundation of China(2021ZZ115); National Natural Science Foundation of China(2021ZR132); Natural Science Foundation of Fujian Province(2021J011073); regional development projects in Fujian Province(2021H4008); Science and Technology Service Network Initiative from the Chinese Academy of Sciences(STS2023T3039)
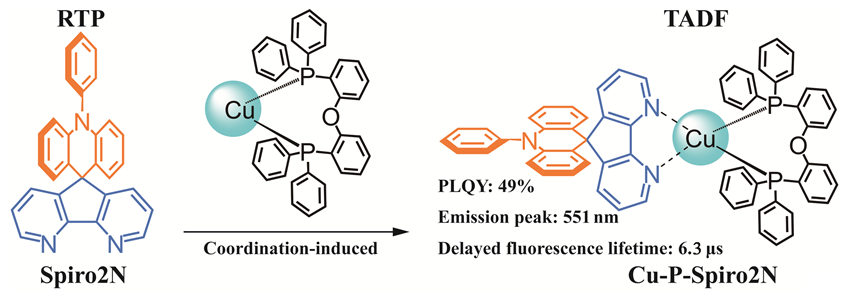
In this study, a Cu(I) complex luminescent material Cu-P-Spiro2N with thermally activated delayed fluorescence (TADF) properties was successfully designed by combining the donor-acceptor (D-A) type ligand 10-phenyl-10H-spiro[acridine-9,9'-(4,5-diazafluorene)] (Spiro2N) with long afterglow emission properties and the auxiliary phosphine ligand bis(2-diphenylphosphinophenyl) ether (POP). The molecular structures of the Cu(I) complex were confirmed by nuclear magnetic resonance spectroscopy, and the crystal structure of the Cu-P-Spiro2N complex was further characterized by X-ray single crystal diffraction. Cu-P-Spiro2N belongs to the triclinic crystal system with cell parameters α=90.14(2)°, β=115.43(3)°, γ=115.55(3)°, a=15.10(6) nm, b=15.15(4) nm, c=16.62(6) nm. Due to the two mutually orthogonal π-conjugated planar structures of the ligand molecule Spiro2N, its highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) are well separated, but with a relatively large singlet-triplet energy gap (∆EST). After coordination of the metal Cu(I) fragment with the ligand Spiro2N, the energy gap between HOMO and LUMO is further reduced, and the charge transfer (CT) state energy level is further lowered, which makes the complex Cu-P-Spiro2N have a very small ∆EST of 0.05 eV. The small ∆EST facilitates the reverse intersystem crossing process, thereby achieving TADF emission. The emission of this complex mainly comes from the D-A ligand Spiro2N, which exhibits metal perturbation intramolecular ligand charge transfer (ILCT) properties. At room temperature, in the doped polymethylmethacrylate (PMMA) film (10% (w)), the Cu-P-Spiro2N complex exhibits strong yellow light emission with an emission peak at 551 nm, a photoluminescence quantum efficiency of 49%, and an excited state lifetime of 6.3 μs. The results of this study indicate that by coordinating with Cu(I) ions, the excited state energy level of the ligand molecule can be modulated, reducing ∆EST and thus achieving TADF emission.
Dengchao Zhang , Jihui Jia , Dong Liang , Xianbao Cai , Yuqing Zhao , Xianglong Hu , Yubing Jiang , Canzhong Lu . Design, Synthesis and Properties of Cu(I) Complexes with a Nitrogen-containing Spirocycle Ligand for Delayed Fluorescence Materials[J]. Acta Chimica Sinica, 2024 , 82(8) : 887 -893 . DOI: 10.6023/A24040148
| [1] | Arias-Rotondo, D. M.; McCusker, J. K. Chem. Soc. Rev. 2016, 45, 5803. |
| [2] | Ma, D.; Tsuboi, T.; Qiu, Y.; Duan, L. Adv. Mater. 2017, 29, 1603253. |
| [3] | Hong, G.; Gan, X.; Leonhardt, C.; Zhang, Z.; Seibert, J.; Busch, J. M.; Br?se, S. Adv. Mater. 2021, 33, 2005630. |
| [4] | Li, X.; Xie, Y.; Li, Z. Chem. Asian J. 2021, 16, 2817. |
| [5] | Ge, F.; Zhang, K.; Cao, Q.; Xu, H.; Zhou, T.; Zhang, W.; Ban, X.; Zhang, X.; Li, N.; Zhu, P. Acta Chim. Sinica 2023, 81, 1157 (in Chinese). |
| [5] | (葛凤洁, 张开志, 曹清鹏, 徐慧, 周涛, 张文浩, 班鑫鑫, 张晓波, 李娜, 朱鹏, 化学学报, 2023, 81, 1157.) |
| [6] | Tao, P.; Zheng, X.; Wang, G.; Sheng, X.; Jiang, H.; Li, W.; Jin, J.; Wong, S.-H.; Miao, Y.; Wang, H.; Wong, W.-Y. Acta Chim. Sinica 2023, 81, 891 (in Chinese). |
| [6] | (陶鹏, 郑小康, 王国良, 盛星浩, 姜贺, 李文桃, 靳继彪, 王瑞鸿, 苗艳勤, 王华, 黄维扬, 化学学报, 2023, 81, 891.) |
| [7] | Kalinowski, J.; Fattori, V.; Cocchi, M.; Williams, J. A. G. Coordin. Chem. Rev. 2011, 255, 2401. |
| [8] | Yersin, H.; Rausch, A. F.; Czerwieniec, R.; Hofbeck, T.; Fischer, T. Coordin. Chem. Rev. 2011, 255, 2622. |
| [9] | Lee, J.; Chen, H. F.; Batagoda, T.; Coburn, C.; Djurovich, P. I.; Thompson, M. E.; Forrest, S. R. Nat. Mater. 2016, 15, 92. |
| [10] | Ren, B.-Y.; Yi, J.-C.; Zhong, D.-K.; Zhao, Y.-Z.; Guo, R.-D.; Sheng, Y.-G.; Sun, Y.-G.; Xie, L.-H.; Huang, W. Acta Chim. Sinica 2020, 78, 56 (in Chinese). |
| [10] | (任保轶, 依建成, 钟道昆, 赵玉志, 郭闰达, 盛永刚, 孙亚光, 解令海, 黄维, 化学学报, 2020, 78, 56.) |
| [11] | Zhu, S.; Huang, X.; Han, X.; Liu, S. Acta Chim. Sinica 2022, 80, 1066 (in Chinese). |
| [11] | (朱诗敏, 黄鑫, 韩勰, 刘思敏, 化学学报, 2022, 80, 1066.) |
| [12] | Cao, L.; Yang, X.; Li, M.; Liu, L.; Yu, J.; Tan, H. Chin. J. Org. Chem. 2022, 42, 1831 (in Chinese). |
| [12] | (曹丽琴, 杨小琴, 李茂秋, 刘琳, 于俊婷, 谭华, 有机化学, 2022, 42, 1831.) |
| [13] | Czerwieniec, R.; Leitl, M. J.; Homeier, H. H. H.; Yersin, H. Coordin. Chem. Rev. 2016, 325, 2. |
| [14] | Li, X.; Zhang, J.; Zhao, Z.; Yu, X.; Li, P.; Yao, Y.; Liu, Z.; Jin, Q. H.; Bian, Z.; Lu, Z. H.; Huang, C. ACS Appl. Mater. Inter. 2019, 11, 3262. |
| [15] | Mohankumar, M.; Holler, M.; Meichsner, E.; Nierengarten, J. F.; Niess, F.; Sauvage, J. P.; Delavaux-Nicot, B.; Leoni, E.; Monti, F.; Malicka, J. M.; Cocchi, M.; Bandini, E.; Armaroli, N. J. Am. Chem. Soc. 2018, 140, 2336. |
| [16] | Hamze, R.; Peltier, J. L.; Sylvinson, D.; Jung, M.; Cardenas, J.; Haiges, R.; Soleilhavoup, M.; Jazzar, R.; Djurovich, P. I.; Bertrand, G.; Thompson, M. E. Science 2019, 363, 601. |
| [17] | Jia, J. H.; Zhang, D. C.; Liang, D.; Wang, Q.; Wang, Z. Q.; Zhang, L.; Lu, C. Z. Adv. Opt. Mater. 2024, 12, 2303053. |
| [18] | Armaroli, N.; Accorsi, G.; Cardinali, F.; Listorti, A. Photochemistry and Photophysics of Coordination Compounds: Copper, Eds.: Balzani, V.; Campagna, S., Springer, Berlin Heidelberg, 2007, pp. 69-115. |
| [19] | Osawa, M.; Kawata, I.; Ishii, R.; Igawa, S.; Hashimoto, M.; Hoshino, M. J. Mater. Chem. C 2013, 1, 4375. |
| [20] | Klein, M.; Rau, N.; Wende, M.; Sundermeyer, J.; Cheng, G.; Che, C.-M.; Schinabeck, A.; Yersin, H. Chem. Mater. 2020, 32, 10365. |
| [21] | Shafikov, M. Z.; Suleymanova, A. F.; Czerwieniec, R.; Yersin, H. Inorg. Chem. 2017, 56, 13274. |
| [22] | Volz, D.; Chen, Y.; Wallesch, M.; Liu, R.; Flechon, C.; Zink, D. M.; Friedrichs, J.; Flugge, H.; Steininger, R.; Gottlicher, J.; Heske, C.; Weinhardt, L.; Brase, S.; So, F.; Baumann, T. Adv. Mater. 2015, 27, 2538. |
| [23] | Jia, J.-H.; Liang, D.; Yu, R.; Chen, X.-L.; Meng, L.; Chang, J.-F.; Liao, J.-Z.; Yang, M.; Li, X.-N.; Lu, C.-Z. Chem. Mater. 2019, 32, 620. |
| [24] | Cai, X. B.; Liang, D.; Yang, M.; Wu, X. Y.; Lu, C. Z.; Yu, R. Chem. Commun. (Camb). 2022, 58, 8970. |
| [25] | Liang, D.; Jia, J.-H.; Cai, X.-B.; Zhao, Y.-Q.; Wang, Z.-Q.; Lu, C.-Z. Inorg. Chem. Front. 2022, 9, 6561. |
| [26] | Liang, D.; Jia, J. H.; Yang, M.; Cai, X. B.; Yu, R.; Lu, C. Z. Adv. Opt. Mater. 2022, 10, 2201130. |
| [27] | Adamo, C.; Barone, V. J. Chem. Phys. 1999, 110, 6158. |
| [28] | Perdew, J. P.; Ernzerhof, M.; Burke, K. J. Chem. Phys. 1996, 105, 9982. |
| [29] | Cances, E.; Mennucci, B.; Tomasi, J. J. Chem. Phys. 1997, 107, 3032. |
| [30] | Mennucci, B.; Cances, E.; Tomasi, J. J. Phys. Chem. 1997, 101, 10506. |
| [31] | Miertu?, S.; Scrocco, E.; Tomasi, J. Chem. Phys. 1981, 55, 117. |
| [32] | Roy, L. E.; Hay, P. J.; Martin, R. L. J. Chem. Theory. Comput. 2008, 4, 1029. |
| [33] | Hariharan, P. C.; Pople, J. A. Mol. Phys. 1974, 27, 209. |
| [34] | Czerwieniec, R.; Kowalski, K.; Yersin, H. Dalton. T. 2013, 42, 9826. |
| [35] | Volz, D.; Wallesch, M.; Grage, S. L.; Gottlicher, J.; Steininger, R.; Batchelor, D.; Vitova, T.; Ulrich, A. S.; Heske, C.; Weinhardt, L.; Baumann, T.; Brase, S. Inorg. Chem. 2014, 53, 7837. |
| [36] | Endo, A.; Sato, K.; Yoshimura, K.; Kai, T.; Kawada, A.; Miyazaki, H.; Adachi, C. Appl. Phys. Lett. 2011, 98, 083302. |
| [37] | Zhang, Q.; Li, J.; Shizu, K.; Huang, S.; Hirata, S.; Miyazaki, H.; Adachi, C. J. Am. Chem. Soc. 2012, 134, 14706. |
| [38] | Yersin, H.; Czerwieniec, R.; Hupfer, A. Organic Photonics V. 2012, 8435, 843508. |
/
| 〈 |
|
〉 |