

Asymmetric Hydrophosphination of Diarylphosphine Oxides to α,β-Unsaturated Bifunctional Compounds Catalyzed by Chiral Oxazaborolidine
Received date: 2024-05-22
Online published: 2024-06-13
Supported by
National Natural Science Foundation of China(22361053); National Natural Science Foundation of China(21961045); Yunnan Fundamental Research Projects(202301AU070125); Yunnan Fundamental Research Projects(202401BC070018); Yunnan Key Laboratory of Chiral Functional Substance Research and Application(202402AN360010); “Yunnan Rejuvenation Talent”
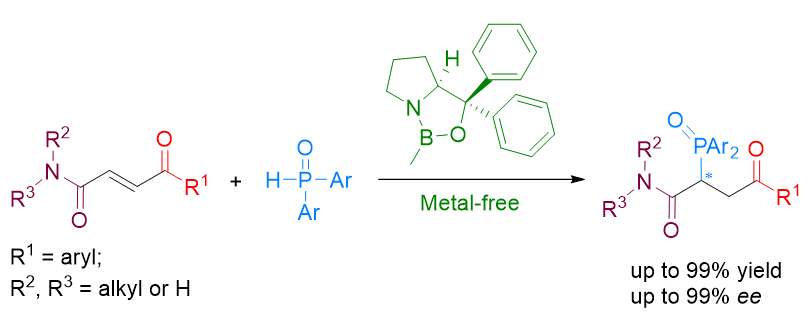
Oxazaborolidines (CBS) have been widely utilized as organocatalysts in enantioselective organic synthesis over the past thirty years, particularly for the asymmetric reduction of ketones and enantioselective cycloaddition reactions. The history of oxazaborolidines functioning as chiral Lewis acids has demonstrated that CBS requires activation by strong protonic acids or Lewis acids to enhance the Lewis acidity of boron, such as TfOH, Tf2NH, and AlBr3. In our previous work, we introduced a new application of CBS in the asymmetric 1,4-addition of diarylphosphine oxides to α,β-unsaturated ketones and esters. Unlike traditional CBS-catalyzed reactions, this catalytic system does not require strong protonic acids or Lewis acids to activate CBS; instead it likely functions as a Lewis pair to cleave the O—H bond and provide a chiral phosphorous intermediate. To further expand the application of this new method, we selected α,β-unsaturated bifunctional compounds bearing both a ketone and an amide group on either side of the double bond as substrates for this study. Initially, several oxazaborolidines and reaction conditions were explored for the model reaction. The results indicated that under optimal conditions (20 mol% Me-CBS at room temperature in acetonitrile for 2 h), the reaction proceeded well with high yield (99%) and high enantiomeric excess (93% ee). Subsequently, various substrates were examined under these optimal conditions yielding products with 52%~99% yields and 63%~99% ee. The general procedure involved stirring a mixture of substrates (0.24 mmol, 1.2 equiv.), diarylphosphine oxides (0.2 mmol, 1 equiv.), and CBS (20 mol% Me-CBS) in 2 mL acetonitrile for 2 h followed by purification via flash column chromatography. Finally, the potential mechanism behind how the catalyst functions as a Lewis pair to control enantioselectivity was discussed. Additionally, the chiral products have potential applications in synthesizing valuable phosphine ligands.
Qi Gao , Lirong Chen , Jinyi Qian , Ruifeng Fan , Weiqing Sun , Yafei Guo , Baomin Fan . Asymmetric Hydrophosphination of Diarylphosphine Oxides to α,β-Unsaturated Bifunctional Compounds Catalyzed by Chiral Oxazaborolidine[J]. Acta Chimica Sinica, 2024 , 82(7) : 742 -747 . DOI: 10.6023/A24050166
| [1] | Corey E. J. Angew. Chem., Int. Ed. 2002, 41, 1650. |
| [2] | (a) Hirao A.; Itsuno S.; Nakahama S.; Yamazaki N. J. Chem. Soc. Chem. Commun. 1981, 315. |
| [2] | (b) Itsuno S.; Ito K.; Hirao A.; Nakahama N. J. Org. Chem. 1984, 49, 555. |
| [3] | (a) Corey E. J.; Bakshi R. K.; Shibata S. J. Am. Chem. Soc. 1987, 109, 5551. |
| [3] | (b) Corey E. J.; Bakshi R. K.; Shibata S.; Chen C. P.; Singh V. K. J. Am. Chem. Soc. 1987, 109, 7925. |
| [4] | Liu D.; Canales E.; Corey E. J. J. Am. Chem. Soc. 2007, 129, 1498. |
| [5] | Corey E. J. Angew. Chem. Int. Ed. 2009, 48, 2100. |
| [6] | (a) Corey E. J.; Loh T. P. J. Am. Chem. Soc. 1991, 113, 8966. |
| [6] | (b) Corey E. J.; Shibata T.; Lee T. W. J. Am. Chem. Soc. 2002, 124, 3808. |
| [6] | (c) Canales E.; Corey E. J. J. Am. Chem. Soc. 2007, 129, 12686. |
| [6] | (d) Zhou G.; Corey E. J. J. Am. Chem. Soc. 2005, 127, 11958. |
| [7] | (a) Zhu R.; Liao K.; Yu J.; Zhou J. Acta Chim. Sinica 2020, 78, 193 (in Chinese). |
| [7] | (朱仁义, 廖奎, 余金生, 周剑, 化学学报, 2020, 78, 193.) |
| [7] | (b) Novas B. T.; Waterman R. ChemCatChem 2022, 14, e202200988. |
| [7] | (c) Zhang Y.; Zhu S. Acta Chim. Sinica 2023, 81, 777 (in Chinese). |
| [7] | (张艳东, 朱守非, 化学学报, 2023, 81, 777.) |
| [7] | (d) Luo C.; Yin Y.; Jiang Z. Chin. J. Org. Chem. 2023, 43, 1963 (in Chinese). |
| [7] | (罗诚, 尹艳丽, 江智勇, 有机化学, 2023, 43, 1963.) |
| [7] | (e) Zhang J.; Ni H.; Wu Q.; Yang J.; Zhang J. Chin. J. Org. Chem. 2022, 42, 3118 (in Chinese). |
| [7] | (张洁明, 倪航, 吴起, 杨俊锋, 张俊良, 有机化学, 2022, 42, 3118.) |
| [8] | (a) Feng J.; Chen X.; Shi M.; Duan W. J. Am. Chem. Soc. 2010, 132, 5562. |
| [8] | (b) Lu Z.; Zhang H.; Yang Z.; Ding N.; Meng L.; Wang J. ACS Catal. 2019, 9, 1457. |
| [8] | (c) Yue W.; Xiao J.; Zhang S.; Yin L. Angew. Chem., Int. Ed. 2020, 59, 7057. |
| [8] | (d) Pérez J. M.; Postolache R.; Castineira Reis M.; Sinnema E. G.; Vargová D.; de Vries F.; Otten E.; Ge L.; Harutyunyan S. R. J. Am. Chem. Soc. 2021, 143, 20071. |
| [8] | (e) Yu X.; Lu L.; Zhang Z.; Shi D.; Xiao W. Org. Chem. Front. 2022, 10, 133. |
| [8] | (f) Sun G.; Xiao F.; Duan W. Chin. J. Org. Chem. 2020, 40, 61 (in Chinese). |
| [8] | (孙贵救, 肖繁花, 段伟良, 有机化学, 2020, 40, 61.) |
| [8] | (g) Zhang F.; Luan Y.; Ye M. Chin. J. Org. Chem. 2021, 41, 3880 (in Chinese). |
| [8] | (张凤萍, 栾玉新, 叶萌春, 有机化学, 2021, 41, 3880.) |
| [9] | (a) Saito B.; Egami H.; Katsuki T. J. Am. Chem. Soc. 2007, 129, 1978. |
| [9] | (b) Yang F.; Zhao D.; Lan J.; Xi P.; Yang L.; Xiang S.; You J. Angew. Chem., Int. Ed. 2008, 47, 5646. |
| [9] | (c) Maiti R.; Yan J.; Yang X.; Mondal B.; Xu J.; Chai H.; Jin Z.; Chi Y. R. Angew. Chem., Int. Ed. 2021, 60, 26616. |
| [9] | (d) Hu H.; Ren X.; He J.; Zhu L.; Fang S.; Su Z.; Wang T. Sci. China Chem. 2022, 65, 2500. |
| [9] | (e) Guo F.; Chen J.; Huang Y. ACS Catal. 2021, 11, 6316. |
| [10] | Qian J.; Zhao H.; Gao Q.; Chen L.; Shi Y.; Li J.; Guo Y.; Fan B. Org. Chem. Front. 2023, 10, 5672. |
| [11] | Shi Y.; Chen L.; Gao Q.; Li J.; Guo Y.; Fan B. Org. Lett. 2023, 25, 6495. |
| [12] | Chen L.; Wang G.; Nong X.; Shao W.; Li J.; Guo Y.; Fan B. Chem. Eur. J. 2024, 30, e202401017. |
| [13] | Lu G.; Xiao L.; Que Q.; Leng T.; Li J.; Guo Y.; Fan B. J. Org. Chem. 2024, 89, 7573. |
/
| 〈 |
|
〉 |