

Electrochromic Electrode Based on Polyaniline and Silver Nanowires Based Localized Surface Plasmon Resonance
Received date: 2024-02-22
Online published: 2024-06-20
Supported by
National Natural Science Foundation of China(21876119)
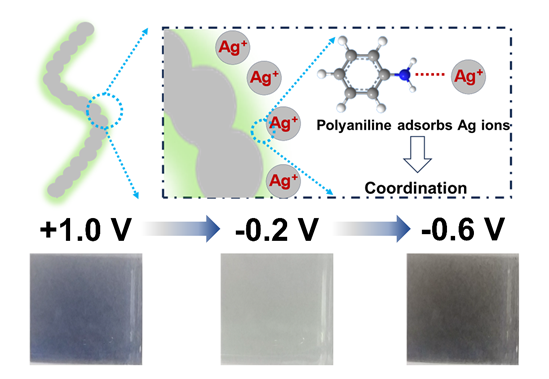
Electrochromism refers to the reversible color change phenomenon under an applied voltage, which demonstrates wide application potential in information displays and optical modulation devices. Conductive polymers, an important type of electrochromic materials, have gained wide attention for their simple preparation process, cost-effectiveness, fast response speed, and good cycling stability. Electrochromic devices utilizing local surface plasmon resonance based on metallic deposition have also attracted great interest. By controlling the reduction of metal cations, the deposition of metal particles or films can be achieved for controlling the device's color or reflection state. Herein, electrochromic nanocomposites combining silver nanowires and polyaniline were synthesized using a self-assembly method to create composite thin film electrodes exhibiting both the electrochromic and local surface plasmon resonance effects. The obtained electrode can satisfy the inherent electrochromic effect of polyaniline itself, which means the reversible variation from light green to deep purple. Meanwhile, under further negative potential, the lower transmittance of nearly 0% of the black state can be achieved. This is because silver nanowires are oxidized into silver ions at a positive potential, and then reduced to elemental silver under the negative potential, absorbing a large amount of light. In addition, different from traditional electrochromic devices based on the single silver ion, the as-prepared electrodes demonstrate reversible modulation due to the adsorption of silver ions by amine groups on aniline, which can effectively reduce the diffusion of silver ions into the electrolyte and thus improving the stability of this combined electrochromic effect. The electrode displays a dark-colored state with the transmittance of 25.4% at a positive potential. Upon application of a reverse potential, the electrode transitions to a faded state (83.3%), and then returning to a dark-colored state (10.7%) at a higher reverse potential. This electrochromic device with dual-colored state, which can not be achieved by conventional strategy, opens up new possibilities in the fields of information display and optical modulation.
Hongchao Peng , Sheng Chen , Bin Yan . Electrochromic Electrode Based on Polyaniline and Silver Nanowires Based Localized Surface Plasmon Resonance[J]. Acta Chimica Sinica, 2024 , 82(7) : 797 -804 . DOI: 10.6023/A24020062
| [1] | Peng H. C.; Pan M. F.; Jiang H.; Huang W. H.; Wang X.; Yang Q.; Chen S.; Yan B. ACS Appl. Mater. Interfaces 2022, 14, 42402. |
| [2] | Lei P. Y.; Wang J. H.; Gao Y.; Hu C. Y.; Zhang S. Y.; Tong X. R.; Wang Z. P.; Gao Y. H.; Cai G. F. Nano-Micro Letters 2023, 15, 34. |
| [3] | Chen Y. N.; Lei J.; Zhai Y. L.; Zhu Z. J.; Wu W. T.; Lu X. Q. Chin. Chem. Lett. 2023, 34, 108305. |
| [4] | Wang C. C.; Jiang X. J.; Cui P.; Sheng M. F.; Gong X. D.; Zhang L. P.; Fu S. H. ACS Appl. Mater. Interfaces 2021, 13, 12313. |
| [5] | Li R.; Ma X. Y.; Li J. M.; Cao J.; Gao H. Z.; Li T. S.; Zhang X. Y.; Wang L. C.; Zhang Q. H.; Wang G.; Hou C. Y.; Li Y. G.; Palacios T.; Lin Y. X.; Wang H. Z.; Ling X. Nat. Commun. 2021, 12, 1587. |
| [6] | Xu J. W.; Zhang Y.; Zhai T. T.; Kuang Z. Y.; Li J.; Wang Y. M.; Gao Z. D.; Song Y. Y.; Xia X. H. ACS Nano 2018, 12, 6895. |
| [7] | Yang L. L.; Feng J. J.; Wang J. N.; Gao Z. D.; Xu J. W.; Mei Y.; Song Y. Y. Chin. Chem. Lett. 2022, 33, 5169. |
| [8] | Kandpal S.; Ghosh T.; Rani C.; Chaudhary A.; Park J.; Lee P. S.; Kumar R. ACS Energy Lett. 2023, 8, 1870. |
| [9] | Qin M. M.; Li X.; Zheng Y. P.; Zhang Y.; Li C. J. Acta Chim. Sinica 2015, 73, 1161 (in Chinese). |
| [9] | (秦咪咪, 李昕, 郑一平, 张焱, 李从举, 化学学报, 2015, 73, 1161.) |
| [10] | Tu L. L.; Jia C. Y.; Weng X. L.; Deng L. J. Acta Chim. Sinica 2010, 68, 2590 (in Chinese). |
| [10] | (涂亮亮, 贾春阳, 翁小龙, 邓龙江, 化学学报, 2010, 68, 2590.) |
| [11] | Cai G. F.; Tu J. P.; Zhou D.; Zhang J. H.; Wang X. L.; Gu C. D. Sol. Energy Mater. Sol. Cells 2014, 122, 51. |
| [12] | Xiong S. X.; Phua S. L.; Dunn B. S.; Ma J.; Lu X. H. Chem. Mater. 2010, 22, 255. |
| [13] | Zhou K. L.; Wang H.; Jiu J. T.; Liu J. B.; Yan H.; Suganuma K. Chem. Eng. J. 2018, 345, 290. |
| [14] | Onodera R.; Tsuboi A.; Nakamura K.; Kobayashi N. J. Soc. Inf. Display 2016, 24, 424. |
| [15] | Araki S.; Nakamura K.; Kobayashi K.; Tsuboi A.; Kobayashi N. Adv. Mater. 2012, 24, OP122. |
| [16] | Tsuboi A.; Nakamura K.; Kobayashi N. Adv. Mater. 2013, 25, 3197. |
| [17] | Peng H. C.; Jiang H.; Tu S. J.; Zhang S. H.; Ren E. H.; Yan B.; Yang Q.; Chen S. Opt. Lett. 2020, 45, 2443. |
| [18] | Xiong C. Y.; Zhang Y. K.; Xu J. Y.; Dang W. H.; Sun X. H.; An M.; Ni Y. H.; Mao J. J. Nano Research 2023, 16, 9471. |
| [19] | Niu B.; Li Z. A.; Luo D.; Ma X. Y.; Yang Q. S.; Liu Y. E.; Yu X. Y.; He X. R.; Qiao Y.; Wang X. Energ. Environ. Sci. 2023, 16, 1662. |
| [20] | Hu B.; Xu J.; Fan Z. J.; Xu C.; Han S. C.; Zhang J. X.; Ma L. B.; Ding B.; Zhuang Z. C.; Kang Q.; Zhang X. G. Adv. Energy Mater. 2023, 13, 2203540. |
| [21] | He Z. W.; Lü Q. F.; Zhang J. Y. ACS Appl. Mater. Interfaces 2012, 4, 369. |
| [22] | Yang S. W.; Zheng J. M.; Wu X. M.; Xu C. Y. Acta Chim. Sinica 2013, 71, 1041 (in Chinese). |
| [22] | (杨树威, 郑建明, 吴星明, 徐春叶, 化学学报, 2013, 71, 1041.) |
| [23] | Chen Z. J.; Shen T. Y.; Zhang M. H.; Xiao X.; Wang H. Q.; Lu Q. R.; Luo Y. L.; Jin Z.; Li C. H. Adv. Funct. Mater. 2024, 35, 2211673. |
| [24] | Zhao L.; Li Y.; Yu M. M.; Peng Y. Y.; Ran F. Adv. Sci. 2023, 10, 2300283. |
| [25] | Zhou Y.; Wang S. C.; Peng J. Q.; Tan Y. T.; Li C. C.; Boey F. Y. C.; Long Y. Joule 2020, 4, 2458. |
| [26] | Peng H. C.; Yan B.; Jiang M. J.; Liu B. C.; Gu Y. C.; Yao G.; Zhang Y.; Ye L. L.; Bai X.; Chen S. J. Mater. Chem. A 2021, 9, 1669. |
/
| 〈 |
|
〉 |