

Study on the Influence of Component and Concentration of CsPbBrxI3-x All-inorganic Perovskite Quantum Dots on Electronic Structure and Fluorescence Properties
Received date: 2024-03-01
Online published: 2024-07-05
Supported by
Natural Science Foundation of Liaoning Province(2022-MS-368); Department of Education Fund of Liaoning Province(LJKMZ20221495)
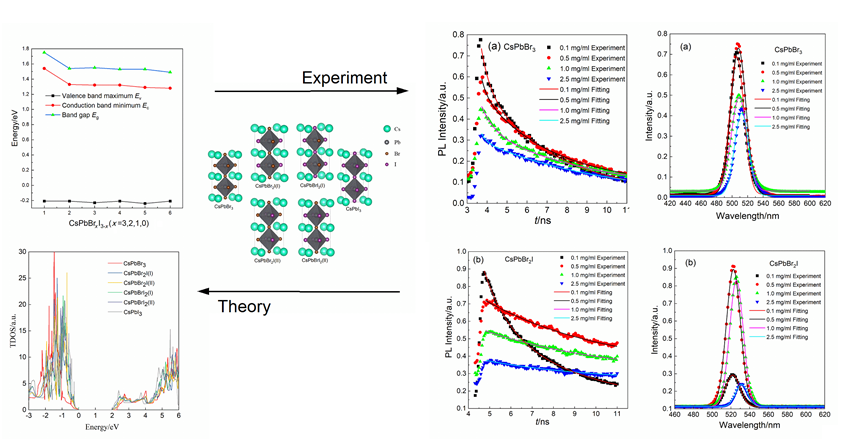
All-inorganic cesium lead halide perovskite materials have broad application prospects in the field of optoelectronics due to their excellent performance. The influences of component and concentration on the electronic structure and fluorescence properties of the CsPbBrxI3-x (x=3, 2, 1, 0) all-inorganic perovskite quantum dots using the first-principles calculations and steady-state and transient fluorescence spectroscopy experiments were investigated. The band structure and density of states of the perovskites CsPbBrxI3-x are calculated to find that the bandgap types are all direct bandgaps and the bandgap becomes narrow with the continuously supplanting of I atoms. This indicates that increasing the I content can achieve the band gap regulation of quantum dots. The steady-state luminescence and picosecond time-correlated single photon counting experiments are completed for CsPbBr3 and CsPbBr2I all-inorganic perovskite quantum dots, it is found that with the increase of quantum dot concentration, the emission spectrum is red-shifted, the photoluminescence intensity first increases and then decreases, and the fluorescence radiation lifetime also increases. These phenomena are mainly caused by the quantum confinement effect and self-absorption effect of quantum dots. As the concentration of quantum dots increases, the size of quantum dots increases due to agglomeration effects, resulting in a red shift in the emission spectrum. When the concentration of quantum dots reaches a certain level, the absorbance of quantum dots gradually saturates, and the self-absorption effect will cause the emission of fluorescence to be absorbed, leading to an increase and then a decrease in fluorescence emission intensity. As the concentration of perovskite quantum dots increases, the quantum confinement effect leads to the size increase of quantum dots due to the aggregation effect, thereby increasing their fluorescence radiation lifetime. With increasing I content, the quantum dot trapping states lead to a distortion of the crystal structure, and thus the fluorescence lifetime becomes short. These results suggest that the exciton recombination process can be controlled by regulating concentration and halogen components.
Yajing Peng , Yuxin Zhao , Jinhui Yang , Xinxin Zhang , Jialing Cheng . Study on the Influence of Component and Concentration of CsPbBrxI3-x All-inorganic Perovskite Quantum Dots on Electronic Structure and Fluorescence Properties[J]. Acta Chimica Sinica, 2024 , 82(8) : 879 -886 . DOI: 10.6023/A24030066
| [1] | Manser, J. S.; Christians, J. A.; Kamat, P. V. Chem. Rev. 2016, 116, 12956. |
| [2] | Zhang, Y. P.; Liu, J. Y.; Wang, Z. Y.; Xue, Y. Z.; Ou, Q. D.; Polavarapu, L.; Zheng, J. L.; Qi, X.; Bao, Q. L. Chem. Commun. 2016, 52, 13637. |
| [3] | Chen, W. W.; Xin, X.; Zang, Z. G.; Tang, X. S.; Li, C. L.; Hu, W.; Zhou, M.; Du, J. J. Solid State Chem. 2017, 255, 115. |
| [4] | Liu, F.; Zhang, Y. H.; Ding, C.; Kobayashi, S.; Izuishi, T; Nakazawa, N.; Toyoda, T.; Ohta, T.; Hayase, S.; Minemoto, T.; Yoshino, K.; Dai, S. Y.; Shen, Q. ACS Nano 2017, 11, 10373. |
| [5] | Liu, M.; Johnston, M. B.; Snaith, H. J. Nature 2013, 501, 395. |
| [6] | Jeon, N. J.; Noh, J. H.; Yang, W. S.; Kim, Y. C.; Ryu, S.; Seo, J.; Seok, S. I. Nature 2015, 517, 476. |
| [7] | Zhang, L.; Lin, S. Sol. Energ. Mat. Sol. C. 2020, 204, 110237. |
| [8] | Wang, J.; Wang, N.; Jin, Y.; Si, J.; Tan, Z. K.; Du, H.; Cheng, L.; Dai, X.; Bai, S.; He, H.; Ye, Z.; Lai, M. L.; Friend, R. H.; Huang, W. Adv. Mater. 2015, 27, 2311. |
| [9] | Chen, H.; Guo, A.; Zhu, J.; Cheng, L.; Wang, Q. Appl. Surf. Sci. 2019, 465, 656. |
| [10] | Xu, Z.; Wang, S.; Hu, X.; Jiang, J.; Wang, L. Solar RRL 2018, 2, 1800204. |
| [11] | Nikl, M.; Nitsch, K.; Polak, K.; Pazzi, G.; Fabeni, P.; Citrin, D.; Gurioli, M. J. Lumin. 1997, 72, 377. |
| [12] | Liang, J.; Wang, C. X.; Wang, Y. R.; Xu, Z. R.; Lu, Z. P.; Ma, Y.; Zhu, H. F.; Hu, Y.; Xiao, C. C.; Yi, X.; Zhu, G. Y.; Lv, H. L.; Ma, L. B.; Chen, T.; Tie, Z. X.; Jin, Z.; Liu, J. J. Am. Chem. Soc. 2016, 138, 15829. |
| [13] | Hu, Z.; Lin, Z.; Su, J.; Zhang, J.; Hao, Y. Solar RRL 2019, 3, 1900304. |
| [14] | Zhang, H.; Jin, Z. J. Semicond. 2021, 42, 4. |
| [15] | Li, M.; Zhang, X.; Du, Y. Y.; Yang, P. J. Lumin. 2017, 190, 397. |
| [16] | Kang, J.; Wang, L. W. J. Phys. Chem. Lett. 2017, 8, 489. |
| [17] | Koscher, B. A.; Swabeck, J. K.; Bronstein, N. D.; Alivisatos, A. P. J. Am. Chem. Soc. 2017, 139, 6566. |
| [18] | Akkerman, Q. A.; D'Innocenzo, V.; Accornero, S.; Scarpellini, A.; Petrozza, A.; Prato, M.; Manna, L. J. Am. Chem. Soc. 2015, 137, 10276. |
| [19] | Parobek, D.; Dong, Y.; Qiao, T.; Rossi, D.; Son, D. H. J. Am. Chem. Soc. 2017, 139, 4358. |
| [20] | Liu, H.; Liu, Z.; Xu, W.; Yang, L.; Liu, Y.; Yao, D.; Zhang, D.; Zhang, H.; Yang, B. ACS Appl. Mater. Interfaces 2019, 11, 14256. |
| [21] | Parobek, D.; Roman, B. J.; Dong, Y. T.; Jin, H.; Lee, E.; Sheldon, M.; Son, D. H. Nano Lett. 2016, 16, 7376. |
| [22] | Milstein, T. J.; Kroupa, D. M.; Gamelin, D. R. Nano Lett. 2018, 18, 3792. |
| [23] | Lee, M. M.; Teuscher, J.; Miyasaka, T.; Murakami, T. N.; Snaith, H. J. Science 2012, 338, 643. |
| [24] | Sun, Q. D.; Yin, W. J. J. Am. Chem. Soc. 2017, 139, 14905. |
| [25] | Divitini, G.; Cacovich, S.; Matteocci, F.; Cinà, L.; Di Carlo, A.; Ducati, C. Nature Energy 2016, 1, 15012. |
| [26] | Protesescu, L.; Nedelcu, G.; Yakunin, S.; Bodnarchuk, M. I.; Grotevent, M. J.; Kovalenko, M. V. Nano Lett. 2015, 15, 3692. |
| [27] | Choi, H.; Jeong, J.; Kim, H. B.; Kim, S.; Walker, B.; Kim, G. H.; Kim, J. Y. Nano Energy 2014, 7, 80. |
| [28] | Eperon, G. E.; Paternò, G. M.; Sutton, R. J.; Zampetti, A.; Haghighirad, A. A.; Cacialli, F.; Snaith, H. J. J. Mat. Chem. A 2015, 3, 19688. |
| [29] | Beal, R. E.; Slotcavage, D. J.; Leijtens, T.; Bowring, A. R.; Belisle, R. A.; Nguyen, W. H.; Burkhard, G. F.; Hoke, E. T.; McGehee, M. D. J. Phys. Chem. Lett. 2016, 7, 746. |
| [30] | Zheng, Y. F.; Yang, X. Y.; Su, R.; Wu, P.; Gong, Q. H.; Zhu, R. Adv. Funct. Mater. 2020, 30, 2000457. |
| [31] | Jaramillo-Quintero, O. A.; Sanchez, R. S.; Rincon, M.; Mora-Sero, I. J. Phys. Chem. Lett. 2015, 6, 1883. |
| [32] | Xu, Y, ; Chen, Q.; Zhang, C.; Wang, R.; Wu, H.; Zhang, X.; Xing, G.; Yu, W. W.; Wang, X.; Zhang, Y.; Xiao, M. J. Am. Chem. Soc. 2016, 138, 3761. |
| [33] | Maleka, P. M.; Dima, R. S.; Ntwaeaborwa, O. M.; Maphanga, R. R. Phys. Scripta 2023, 98, 045505. |
| [34] | Bl?chl, P. E. Phys. Rev. B 1994, 50, 17953. |
| [35] | Perdew, J. P.; Burke, K.; Ernzerh, M. Phys. Rev. Lett. 1996, 77, 3865. |
| [36] | Murtaza, G.; Ahmad, I. Phys. B Condens. Matter 2011, 406, 3222. |
| [37] | Woods-Robinson, R.; Horton, M. K.; Persson, K. A. Patterns 2023, 4, 100823. |
| [38] | Chen, Y.; Shi, T.; Liu, P.; Xie, W.; Chen, K.; Xu, X.; Wang, X. J. Mater. Chem. A 2019, 7, 20201. |
| [39] | Trots, D. M.; Myagkota, S. V. J. Phys. Chem. Solids 2008, 69, 2520. |
| [40] | Maughan, A. E.; Ganose, A. M.; Scanlon, D. O.; Almaker, M. A. Chem. Mater. 2019, 31, 1184. |
| [41] | Yang, Z.; Rajagopal, A.; Jen, A. K. Y. Adv. Mater. 2017, 29, 1704418. |
| [42] | Eperon, G. E.; Stranks, S. D.; Menelaou, C.; Johnston, M. B.; Herz, L. M.; Snaith, H. J. Energy Environ. Sci. 2014, 7, 982. |
| [43] | Rajeswarapalanichamy, R.; Amudhavalli, A.; Padmavathy, R.; Iyakutti, K. Mater. Sci. Eng. B 2020, 258, 114560. |
| [44] | Xu, S.; Libanori, A.; Luo, G.; Chen, J. Iscience 2021, 24, 102235. |
| [45] | Ahmad, M.; Rehman, G.; Ali, L.; Shafiq, M.; Iqbal, R.; Ahmad, R.; Khan, T.; Jalali-Asadabadi, S.; Maqbool, M.; Ahmad, I. J. Alloys Compd. 2017, 705, 828. |
| [46] | Caicedo‐Dávila, S.; Caprioglio, P.; Lehmann, F.; Levcenco, S.; Stolterfoht, M.; Neher, D.; Kronik, L.; Abou‐Ras, D. Adv. Funct. Mater. 2023, 33, 2305240. |
| [47] | Zhang, Y.; Li, Y.; Liu, Y.; Li, H.; Fan, J. Appl. Surf. Sci. 2019, 466, 119. |
| [48] | Naghadeh, S. B.; Luo, B.; Pu, Y. C.; Schwartz, Z.; Hollingsworth, W. R.; Lindley, S. A.; Brewer, A. S.; Ayzner, A. L.; Zhang, J. Z. J. Phys. Chem. C 2019, 123, 4610. |
| [49] | Zhong, M.; Zhao, Z.; Luo, Y.; Zhou, F.; Peng, Y.; Yin, Y.; Zhou, W.; Tang, D. RSC Adv. 2020, 10, 18368. |
| [50] | Qiao, T.; Son, D. H. Acc. Chem. Res. 2021, 54, 1399. |
| [51] | Wang, Y.; Yang, Y.; Wang, P.; Bai, X. Optik 2017, 139, 56. |
| [52] | Toci, G.; Alderighi, D.; Pirri, A.; Vannini, M. Appl. Phys. B 2012, 106, 73. |
| [53] | Zhou, N.; Yuan, M.; Gao, Y.; Li, D.; Yang, D. ACS Nano 2016, 10, 4154. |
| [54] | Kim, D. W.; Hyun, C.; Shin, T. J.; Jeong, U. ACS Nano 2022, 16, 2521. |
| [55] | Liu, X. F.; Zou, L.; Yang, C.; Zhao, W.; Li, X. Y.; Sun, B.; Hu, C. X.; Yu, Y.; Wang, Q.; Zhao, Q.; Zhang, H. L. ACS Appl. Mater. Interfaces 2020, 12, 43073. |
| [56] | Kim, S.; Park, K. D.; Lee, H. Energies 2021, 14, 275. |
| [57] | Seth, S.; Mondal, N.; Patra, S.; Samanta, A. J. Phys. Chem. Lett. 2016, 7, 266. |
/
| 〈 |
|
〉 |