

The Induced Adsorption of PbI2 Particles on the SnCl2-Sensitized TiO2 Surface
Received date: 2024-04-10
Online published: 2024-07-22
Supported by
National Natural Science Foundation of China(52476073)
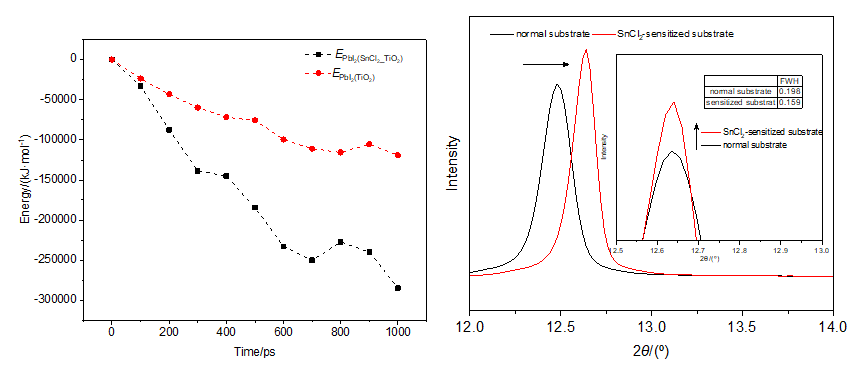
In recent years, perovskite solar cells have emerged as a bright star in the realm of renewable energy, capturing the attention of researchers and industry experts with their high conversion efficiencies, low costs, and exceptional flexibility. Despite these advantages, the Achilles' heel of perovskite solar cells lies in the delicate art of film preparation. The challenges inherent in this process, if not overcome, could potentially hinder the technology's march towards commercialization. This paper delves into a critical aspect of perovskite film formation—the deposition of the PbI2 seed layer in the two-step synthesis of perovskite materials. The seed layer, serving as the cornerstone for the growth of the perovskite lattice, plays a decisive role in the ultimate performance of the solar cell. Inspired by the role of SnCl2 in the silver mirror reaction, this paper proposes the use of an SnCl2 sensitization layer to enhance the adsorption capacity of PbI2 particles on TiO2 substrates. To validate this hypothesis, we conducted a series of molecular dynamics simulations. These simulations provided a microscopic perspective on the deposition process, contrasting the adsorption and deposition behaviors of PbI2 particles on pristine TiO2 surfaces with those on TiO2 surfaces pre-treated with SnCl2. The findings indicated that the substrate sensitized with SnCl2 could significantly reduce the energy of PbI2 particles during the deposition process, facilitating their adsorption and deposition on the substrate. To further analyze the binding energies among SnCl2, PbI2, and TiO2, additional simulations focused on their interactions. These simulations confirmed that SnCl2 could act as an effective bridge, promoting a tighter bond between PbI2 and TiO2. Complementing the simulations, experimental validation was carried out through PbI2 solution deposition. The resulting films, analyzed using scanning electron microscopy (SEM) and X-ray diffraction (XRD), confirmed the positive impact of the SnCl2 sensitization layer on the quality of PbI2 film deposition.
Key words: perovskite solar cells; film; "bridge" particles; induced adsorption
Xinyu Wang , Xiongwen Xu . The Induced Adsorption of PbI2 Particles on the SnCl2-Sensitized TiO2 Surface[J]. Acta Chimica Sinica, 2024 , 82(8) : 865 -870 . DOI: 10.6023/A24040124
| [1] | Wei, J.; Zhao, Q.; Li, H.; Shi, C.-L.; Tian, J.-J.; Cao, G.-Z.; Yu, D.-P. Sci. China Technol. Sci. 2014, 44, 801 (in Chinese). |
| [1] | (魏静, 赵清, 李恒, 施成龙, 田建军, 曹国忠, 俞大鹏, 中国科学: 技术科学, 2014, 44, 801.) |
| [2] | Green, M. A. Prog. Photovolt: Res. Appl. 2009, 17, 183. |
| [3] | Jackson, P.; Hariskos, D.; Lotter, E.; Paetel, S.; Wuerz, R.; Menner, R.; Wischmann, W.; Powalla, M. Photovolt: Res. Appl. 2011, 19, 894. |
| [4] | O'Regan, B.; Gratzel, M. Nature 1991, 353, 737. |
| [5] | Hardin, B. E.; Snaith, H. J.; McGehee, M. D. Nat. Photonics 2013, 6, 162. |
| [6] | Seo, J. H.; Gutacker, A.; Sun, Y. M.; Wu, H. B.; Huang, F.; Cao, Y.; Scherf, U.; Heeger, A. J.; Bazan, G. C. J. Am. Chem. Soc. 2011, 133, 8416. |
| [7] | Deb, S. K. Sol. Energy Mater. Sol. Cells. 2005, 88, 1. |
| [8] | Anaraki, E. H.; Kermanpur, A.; Steier, L.; Domanski, K.; Matsui, T.; Tress, W.; Saliba, M.; Abate, A.; Gr?tzel, M.; Hagfeldt, A.; Correa- Baena, J. P. Energy Environ. Sci. 2016, 9, 3128. |
| [9] | Qiu, L. B.; Deng, J.; Lu, X.; Yang, Z. B.; Peng, H. S. Angew. Chem. Int. Ed. 2014, 53, 10425. |
| [10] | Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. J. Am. Chem. Soc. 2009, 131, 6050. |
| [11] | Pan, T.; Zhou, W.; Wei, Q.; Peng, Z. J.; Wang, H.; Jiang, X. Y.; Zang, Z. H.; Li, H. S.; Yu, D. N.; Zhou, Q. L.; Pan, M. L.; Zhou, W. J.; Ning, Z. J. Adv. Mater. 2023, 35, 2208522. |
| [12] | Lee, J. W.; Lee, D. K.; Jeong, D. N.; Park, N. G. Adv. Funct. Mater. 2019, 29, 1807047. |
| [13] | Chen, H. N.; Wei, Z. H.; He, H. X.; Zheng, X. L.; Wong, K. S.; Yang, S. H. Adv. Energy. Mater. 2016, 6, 1502087. |
| [14] | Tidhar, Y.; Edri, E.; Weissman, H.; Zohar, D.; Hodes, G.; Cahen, D.; Rybtchinski, B.; Kirmayer, S. J. Am. Chem. Soc. 2014, 136, 13249. |
| [15] | Huang, Z. Q.; Duan, X. P.; Zhang, Y.; Hu, X. T.; Tan, L. C.; Chen, Y. W. Nat. Energy. 2016, 155, 166. |
| [16] | Thanh, N. T. K.; Maclean, N.; Mahiddine, S. Chem. Rev. 2014, 114, 7610. |
| [17] | Deng, Y. H.; Zheng, X. P.; Bai, Y.; Wang, Q.; Zhao, J. J.; Huang, J. S. Nat. Energy 2018, 3, 560. |
| [18] | Ryu, S.; Noh, J. H.; Jeon, N. J.; Kim, Y. C.; Yang, S.; Seo, J. W.; Seok, S. I. Energy. Environ. Sci. 2014, 7, 2614. |
| [19] | Stranks, S. D.; Eperon, G. E.; Grancini, G.; Menelaou, C.; Alcocer, M. J. P.; Leijtens, T.; Herz, L. M.; Petrozza, A.; Snaith, H. J. Science 2013, 342, 341. |
| [20] | Zhou, Y. Y.; Yang, M. J.; Wu, W. W.; Vasiliev, A. L.; Zhu, K.; Padture, N. P. J. Mater. Chem. A 2015, 3, 8178. |
| [21] | Carvajal, J. J.; Pujol, M. C.; Díaz, F. In Springer handbook of crystal growth, Vol. 39, Eds.: Dhanaraj, G.; Byrappa, K.; Prasad, V.; Dudley, M., Springer, Berlin, 2010, Chapter C.21. |
| [22] | Zhumekenov, A. A.; Burlakov, V. M.; Saidaminov, M. I.; Alofi, A.; Hague, M. A.; Turedi, B.; Davaasuren, B.; Dursun, I.; Cho, N.; El-Zohry, A. M.; De Bastiani, M.; Giugni, A.; Torre, B.; Di Fabrizio, E.; Mohammed, O. F.; Rothenberger, A.; Wu, T.; Goriely, A.; Bakr, O. M. ACS Energy. Lett. 2017, 2, 1782. |
| [23] | Nayak, P. K.; Moore, D. T.; Wenger, B.; Nayak, S.; Haghighirad, A. A.; Fineberg, A.; Noel, N. K.; Reid, O. G.; Rumbles, G.; Kukura, P.; Vincent, K. A.; Snaith, H. J. Nat. Commun. 2016, 7, 13303. |
| [24] | Xu, Q.; Wang, J.; Shao, W.; Ouyang, X.; Wang, X.; Zhang, X.; Guo, Y. Nanoscale 2020, 17, 9727. |
| [25] | Yu, Y. H.; Xu, X. W.; Liu, J. P.; Liu, Y. H.; Cai, W. H.; Chen, J. X. Surf. Sci. 2021, 714, 121916. |
| [26] | Liang, S.-S. In China Glass Industry Annual Conference and Technical Symposium, Eds.: China Architectural and Industrial Glass Association, 2006, pp. 289-294 (in Chinese). |
| [26] | (梁水生, 中国建筑玻璃与工业玻璃协会, 中国玻璃行业年会暨技术研讨会, 2006, pp. 289-294.) |
| [27] | Park, J. H.; Aluru, N. R. Mol. Simulat. 2009, 35, 31. |
| [28] | Rappe, A. K.; Casewit, C. J.; Colwell, K. S.; Goddard, W. A., III; Skiff, W. M. J. Am. Chem. Soc. 1992, 114, 10024. |
| [29] | Mayo, S. L.; Olafson, B. D.; Goddard, W. A., III. J. Phys. Chem. 1990, 94, 8897. |
/
| 〈 |
|
〉 |