

Syntheses of Sodium and Potassium Complexes Based on Pyridine-2,6-diyl-bis(methylene)-bridged Bis(aminophenolate) Ligands and Catalytic Ring-opening Polymerization of rac-Lactide
Received date: 2024-08-13
Online published: 2024-09-11
Supported by
National Natural Science Foundation of China(21871082)
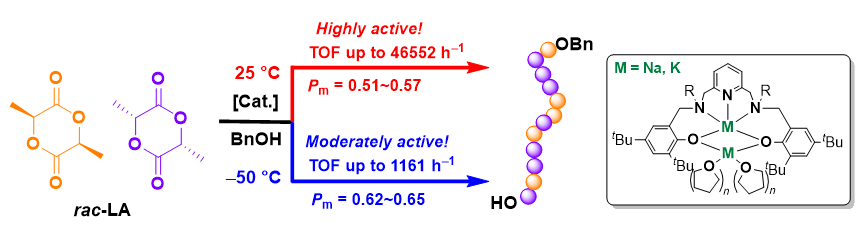
In this work, four novel binuclear sodium and potassium complexes bearing pyridine-2,6-diyl-bis(methylene)- bridged bis(aminophenolate) ligands were synthesized via the reactions of corresponding bisphenol proligands L1H2~L3H2 with excess NaH or KH in tetrahydrofuran at room temperature. All the newly synthesized proligands and complexes were characterized by 1H NMR, 13C NMR and high-resolution mass spectrometry (HRMS) /or elemental analysis. The X-ray diffraction analysis of complex Na2 being obtained as a pair of racemic isomers shows that the molecule possesses two non-symmetrically coordinated sodium centers, with one sodium center five-coordinated by all of the heteroatoms of the multidentate ligand, the other one four-coordinated by two tetrahydrofuran molecules in addition to the two phenolate oxygen atoms, and the two sodium centers are bridged via two phenolate oxygen atoms, leading to a Na•••Na distance of 0.3041 nm. Without the addition of alcohol, the catalytic activity of Na1 towards the ring-opening polymerization (ROP) of rac-lactide (rac-LA) was very low (turnover frequency (TOF)=225 h-1), and the molecular weight of the obtained polymer was much lower than the theoretical one. When benzyl alcohol was used as an initiator, all these sodium and potassium complexes showed high catalytic activities and low isoselectivities of Pm=0.51~0.57 towards the polymerization of rac-LA. The electron-donating effect of the substituents on the skeleton nitrogen atoms of the ligand is beneficial to the improvement of catalytic activity of the corresponding complex, while increasing the steric bulkiness of those substituents is unfavorable to the reaction. Among them, complex Na3 with tert-butyl groups substituted on the skeleton nitrogen atoms showed the highest catalytic activity in the presence of four equiv. of benzyl alcohol (TOF up to 46552 h-1). In addition, with the decrease of the polymerization temperature, the selectivity of complexes for rac-LA polymerization gradually increased. For example, complex Na1 showed a low isoselectivity of Pm=0.57 at room temperature, which could be improved to Pm=0.65 when the polymerization was carried at -50 ℃. The 1H NMR tracking experiments and matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) analysis indicated that, in the absence of benzyl alcohol, the reaction of typical complex Na1 with 20 equiv. of rac-LA afforded cyclic polymers, which is proposed to be formed via an anionic polymerization pathway initiated by the anion generated through the abstraction of lactide methine proton by complex Na1. The NMR scale reactions of complex Na1 with 1 to 4 equiv. of benzyl alcohol supported the presence of weak interactions between Na1 and BnOH instead of the direct alcoholysis reaction; upon further treated with 20 equiv. of rac-LA, active propagation PLA chain end-capped by benzyloxy group at one end could be identified mainly, which was companied by partial decomposition of Na1. On the basis of NMR tracking experiments as well as MALDI-TOF mass spectrometry analysis, it is proposed that, in the presence of benzyl alcohol, the polymerization of rac-LA catalyzed by Na1 processes via ligand assisted monomer activation pathway mainly and the anionic pathway is inhibited partially.
Jingyan Wang , Haiyan Ma . Syntheses of Sodium and Potassium Complexes Based on Pyridine-2,6-diyl-bis(methylene)-bridged Bis(aminophenolate) Ligands and Catalytic Ring-opening Polymerization of rac-Lactide[J]. Acta Chimica Sinica, 2024 , 82(10) : 1058 -1068 . DOI: 10.6023/A24080237
| [1] | Garlotta, D. J. Polym. Environ. 2001, 9 63. |
| [2] | Fukushima, K.; Kimura, Y. Polym. Int. 2006, 55 626. |
| [3] | Thomas, C. M. Chem. Soc. Rev. 2010, 39 165. |
| [4] | Dijkstra, P. J.; Du, H.; Feijen, J. Polym. Chem. 2011, 2 520. |
| [5] | Thomas, C.; Lutz, J.-F. Angew. Chem. Int. Ed. 2011, 50 9244. |
| [6] | Brizzolara, D.; Cantow, H.-J.; Diederichs, K.; Keller, E.; Domb, A. J. Macromolecules 1996, 29 191. |
| [7] | Albertsson, A.-C.; Varma, I. K. Biomacromolecules 2003, 4 1466. |
| [8] | Pongpanit, T.; Saeteaw, T.; Chumsaeng, P.; Chasing, P.; Phomphrai, K. Inorg. Chem. 2021, 60 17114. |
| [9] | Vink, E. T. H.; Davies, S. Ind. Biotech. 2015, 11 167. |
| [10] | Auras, R.; Harte, B.; Selke, S. Macromol. Biosci. 2004, 4 835. |
| [11] | Hofmann, D.; Entrialgo-Casta?o, M.; Kratz, K.; Lendlein, A. Adv. Mater. 2009, 21 3237. |
| [12] | Zhang, G.-D.; Yang, J.-Y.; Feng, X.-D.; Gu, Z.-W. Prog. Chem. 2000, 12 89 (in Chinese). |
| [12] | (张国栋, 杨纪元, 冯新德, 顾忠伟, 化学进展, 2000, 12 89.) |
| [13] | Le Borgne, A.; Vincens, V.; Jouglard, M.; Spassky, N. Makromol. Chem. 2011, 73 37. |
| [14] | Zhong, Z.; Dijkstra, P. J.; Feijen, J. Angew. Chem. Int. Ed. 2002, 41 4510. |
| [15] | Tang, Z.; Chen, X.; Pang, X.; Yang, X.; Zhang, X.; Jing, X. Biomacromolecules 2004, 5 965. |
| [16] | Nomura, N.; Ishii, R.; Yamamoto, Y.; Kondo, T. Chem.-Eur. J. 2007, 13 4433. |
| [17] | Chen, H.-L.; Dutta, S.; Huang, P.-Y.; Lin, C.-C. Organometallics 2012, 31 2016. |
| [18] | Maudoux, N.; Roisnel, T.; Dorcet, V.; Carpentier, J.-F. Chem.-Eur. J. 2014, 20 6131. |
| [19] | Myers, D.; White, A. J. P.; Forsyth, C. M.; Sarazin, Y. Angew. Chem. Int. Ed. 2017, 56 5277. |
| [20] | Yuntawattana, N.; Mcguire, T. M.; Durr, C. B.; Buchard, A.; Williams, C. K. Catal. Sci. Technol. 2020, 10 7226. |
| [21] | Pang, X.; Duan, R.; Li, X.; Hu, C.; Wang, X.; Chen, X. Macromolecules 2018, 51 906. |
| [22] | Wan, Y.; Bai, Y.; He, J.; Zhang, Y. Macromol. Rapid. Comm. 2020, 42 2000491. |
| [23] | Bhattacharjee, J.; Peters, M.; Bockfeld, D.; Tamm, M. Chem.-Eur. J. 2021, 27 5913. |
| [24] | Cheng, M.; Attygalle, A. B.; Lobkovsky, E. B.; Coates, G. W. J. Am. Chem. Soc. 1999, 121 11583. |
| [25] | Malcolm, H.; Chisholm, J. C. G.; Zhen, H. Inorg. Chem. 2001, 40 5051. |
| [26] | Williams, C. K.; Brooks, N. R.; Hillmyer, M. A.; Tolman, W. B. Chem. Commun. 2002, 18 2132. |
| [27] | Malcolm, H.; Chisholm, J. C. G.; Khamphee, P. Inorg. Chem. 2004, 43 6717. |
| [28] | Wu, J.-C.; Huang, B.-H.; Hsueh, M.-L.; Lai, S.-L.; Lin, C.-C. Polymer 2005, 46 9784. |
| [29] | Abbina, S.; Du, G. ACS Macro Lett. 2014, 3 689. |
| [30] | Wang, H.; Ma, H. Chem. Commun. 2013, 49 8686. |
| [31] | Kan, C.; Hu, J.; Huang, Y.; Wang, H.; Ma, H. Macromolecules 2017, 50 7911. |
| [32] | Hu, J.; Kan, C.; Wang, H.; Ma, H. Macromolecules 2018, 51 5304. |
| [33] | Gong, Y.; Ma, H. Chem. Commun. 2019, 55 10112. |
| [34] | Ma, H.; Jiang, X. CN111362885. 2020, 173 487229] |
| [34] | (马海燕, 蒋旭敏, CN111362885. 2020, 173 487229]). |
| [35] | Ma, H.; Shao, M. CN113264901. 2021, 176 93892] |
| [35] | (马海燕, 邵猛, CN113264901. 2021, 176 93892]). |
| [36] | Ma, H.; Gong, S. CN114349781. 2022, 178 368641] |
| [36] | (马海燕, 龚闪闪, CN114349781. 2022, 178 368641]). |
| [37] | Bhattacharjee, J.; Harinath, A.; Nayek, H. P.; Sarkar, A.; Panda, T. K. Chem.-Eur. J. 2017, 23 9319. |
| [38] | Rosen, T.; Rajpurohit, J.; Lipstman, S.; Venditto, V.; Kol, M. Chem.-Eur. J. 2020, 26 17183. |
| [39] | Chellali, J. E.; Alverson, A. K.; Robinson, J. R. ACS Catal. 2022, 12 5585. |
| [40] | Liu, J.-Y.; Liu, S.; Suo, H.-Y.; Qin, Y.-S. Acta Polym. Sin. 2024, 55 770 (in Chinese). |
| [40] | (刘娇玉, 刘爽, 索泓一, 秦玉升, 高分子学报, 2024, 55 770.) |
| [41] | Ma, H.; Spaniol, T. P.; Okuda, J. Angew. Chem. Int. Ed. 2006, 118 7982. |
| [42] | Buffet, J. C.; Kapelski, A.; Okuda, J. Macromolecules 2010, 43 10201. |
| [43] | Liu, X.; Shang, X.; Tang, T.; Hu, N.; Pei, F.; Cui, D.; Chen, X.; Jing, X. Organometallics 2007, 26 2747. |
| [44] | Luo, Y.; Li, W.; Lin, D.; Yao, Y.; Zhang, Y.; Shen, Q. Organometallics 2010, 29 3507. |
| [45] | Yang, S.; Du, Z.; Zhang, Y.; Shen, Q. Chem. Commun. 2012, 48 9780. |
| [46] | Bouyahyi, M.; Ajellal, N.; Kirillov, E.; Thomas, C. M.; Carpentier, J.-F. Chem.-Eur. J. 2011, 17 1872. |
| [47] | Nie, K.; Fang, L.; Yao, Y.; Zhang, Y.; Shen, Q.; Wang, Y. Inorg. Chem. 2012, 51 11133. |
| [48] | Xu, T.-Q.; Yang, G.-W.; Liu, C.; Lu, X. Macromolecules 2017, 50 515. |
| [49] | Duan, Y.-L.; Guo, Q.; Liu, G.-Y.; Yi, Z.-Z.; Feng, S.-P.; Huang, Y. Polym. Chem. 2022, 13 4249. |
| [50] | Ko, B.-T.; Lin, C.-C. J. Am. Chem. Soc. 2001, 123 7973. |
| [51] | Hsueh, M.-L.; Huang, B.-H.; Wu, J.; Lin, C.-C. Macromolecules 2005, 38 9482. |
| [52] | Chen, H.-Y.; Zhang, J.; Lin, C.-C.; Reibenspies, J.-H.; Miller, S.-A. Green Chem. 2007, 9 1038. |
| [53] | Huang, Y.; Tsai, Y.-H.; Hung, W.-C.; Lin, C.-S.; Wang, W.; Huang, J.-H.; Dutta, S.; Lin, C.-C. Inorg. Chem. 2010, 49 9416. |
| [54] | Huang, Y.; Wang, W.; Lin, C.-C.; Blake, M. P.; Clark, L.; Schwarz, A. D.; Mountford, P. Dalton Trans. 2013, 42 9313. |
| [55] | García-valle, F. M.; Estivill, R.; Gallegos, C.; Mosquera, M. E. G.; Tabernero, V.; Cano, J. Organometallics 2015, 34 477. |
| [56] | Dean, R. K.; Reckling, A. M.; Chen, H.; Dawe, L. N.; Schneider, C. M.; Kozak, C. M. Dalton Trans. 2013, 42 3504. |
| [57] | Alhashmialameer, D.; Ikpo, N.; Collins, J.; Dawe, L. N.; Hattenhauer, K.; Kerton, F. M. Dalton Trans. 2015, 44 20216. |
| [58] | Yao, C.; Yang, Y.; Xu, S.; Ma, H. Dalton Trans. 2017, 46 6087. |
| [59] | Ma, H.; Hu, J.; Huang, Y. CN108047256. 2018, 169 46195] |
| [59] | (马海燕, 胡建文, 黄洋, CN108047256. 2018, 169 46195]). |
| [60] | Ma, H.; Li, H.; Huang, Y. CN116854712. 2023, 185 211761] |
| [60] | (马海燕, 李和华, 黄洋, CN116854712. 2023, 185 211761]). |
| [61] | Ma, H.; Huang, Y.; Li, H. CN116874414. 2023, 185 30640] |
| [61] | (马海燕, 黄洋, 李和华, CN116874414. 2023, 185 30640]). |
| [62] | Zhang, J.; Xiong, J.; Sun, Y.; Dai, Z.; Pan, X.; Wu, J. Macromolecules 2014, 47 7789. |
| [63] | Xiong, J.; Zhang, J.; Sun, Y.; Xiong, J.; Pan, X.; Wu, J. Inorg. Chem. 2015, 54 1737. |
| [64] | Dai, Z.; Sun, Y.; Xiong, J.; Pan, X.; Tang, N.; Wu, J. ACS Macro Lett. 2015, 4 556. |
| [65] | Sun, Y.; Xiong, J.; Dai, Z.; Pan, X.; Tang, N.; Wu, J. Inorg. Chem. 2015, 55 136. |
| [66] | Dai, Z.; Sun, Y.; Xiong, J.; Pan, X.; Tang, N.; Wu, J. Catal. Sci. Technol. 2016, 6 515. |
| [67] | Chen, C.; Cui, Y.; Mao, X.; Pan, X.; Wu, J. Macromolecules 2016, 50 83. |
| [68] | Cui, Y.; Chen, C.; Sun, Y.; Wu, J.; Pan, X. Inorg. Chem. Front. 2017, 4 261. |
| [69] | Chen, C.; Jiang, J.; Mao, X.; Cong, Y.; Cui, Y.; Pan, X.; Wu, J. Inorg. Chem. 2018, 57 3158. |
| [70] | Wu, B.-B.; Tian, L.-L.; Wang, Z.-X. RSC Adv. 2017, 7 24055. |
| [71] | Fernández-Millán, M.; Ortega, P.; Cuenca, T.; Cano, J.; Mosquera, M. E. G. Organometallics 2020, 39 2278. |
| [72] | Cui, Y.; Jiang, J.; Mao, X.; Wu, J. Inorg. Chem. 2019, 58 218. |
| [73] | Li, X.; Jia, Z.; Pan, X.; Wu, J. Chem. Asian J. 2019, 14 662. |
| [74] | Harinath, A.; Bhattacharjee, J.; Sarkar, A.; Panda, T. K. New J. Chem. 2019, 43 8882. |
| [75] | Ren, F.; Li, X.; Xian, J.; Han, X.; Cao, L.; Pan, X.; Wu, J. J. Polym. Sci. 2022, 60 2847. |
| [76] | Stopper, A.; Press, K.; Okuda, J.; Goldberg, I.; Kol, M. Inorg. Chem. 2014, 53 9140. |
| [77] | Jeong, Y.; Shin, M.; Seo, M.; Kim, H. Organometallics 2022, 41 328. |
| [78] | Stewart, J. A.; Mckeown, P.; Driscoll, O. J.; Mahon, M. F.; Ward, B. D.; Jones, M. D. Macromolecules 2019, 52 5977. |
| [79] | Marin, P.; Tschan, M. J. L.; Isnard, F.; Robert, C.; Haquette, P.; Trivelli, X.; Chamoreau, L. M.; Guérineau, V.; Rosal, I. D.; Maron, L.; Venditto, V.; Thomas, C. Angew. Chem. Int. Ed. 2019, 58 12585. |
| [80] | Lee, J.; Nayab, S.; Kumar, A.; Kim, D.; Jung, H.; Lee, S.-H.; Cho, D.; Lee, H. Bull. Korean Chem. Soc. 2024, 45 317. |
| [81] | Baker, C. A.; Romain, C.; Long, N. J. Chem. Commun. 2021, 57 12524. |
| [82] | Liu, S.; Li, H.; Zhao, N.; Li, Z. ACS Macro Lett. 2018, 7 624. |
| [83] | Liu, Y.; Zhang, J.; Kou, X.; Liu, S.; Li, Z. ACS Macro Lett. 2022, 11 1183. |
| [84] | Zaky, M. S.; Wirotius, A.-L.; Coulembier, O.; Guichard, G.; Taton, D. ACS Macro Lett. 2022, 11 1148. |
| [85] | Zaky, M. S.; Guichard, G.; Taton, D. Macromolecules 2023, 56 3607. |
| [86] | Kou, X.-H.; Shen, Y.; Li, Z.-B. Acta Polym. Sin. 2020, 51 1121 (in Chinese). |
| [86] | (寇新慧, 沈勇, 李志波, 高分子学报, 2020, 51 1121.) |
| [87] | Li, H.-Q.; Wang, J.-Y.; Wu, L.; Liu, W.; Cheng, R.-H.; Liu, B.-P. Acta Polym. Sin. 2019, 50 1290 (in Chinese). |
| [87] | (李海强, 汪婧怡, 武莉, 刘威, 程瑞华, 刘柏平, 高分子学报, 2019, 50 1290.) |
| [88] | Ghosh, S.; Schulte, Y.; W?lper, C.; Tjaberings, A.; Gr?schel, A. H.; Haberhauer, G.; Schulz, S. Organometallics 2022, 41 2698. |
| [89] | Kremer, A. B.; Mehrkhodavandi, P. Coord. Chem. Rev. 2019, 380 35. |
| [90] | Williams, C. K.; Breyfogle, L. E.; Choi, S. K.; Nam, W.; Young Jr, V. G.; Hillmyer, M. A.; Tolman, W. B. J. Am. Chem. Soc. 2003, 125 11350. |
| [91] | Gruszka, W.; Walker, L. C.; Shaver, M. P.; Garden, J. A. Macromolecules 2020, 53 4294. |
| [92] | Jadrich, C. N.; Pane, V. E.; Lin, B.-H.; Jones, G. O.; Hedrick, J. L.; Park, N. H.; Waymouth, R. M. J. Am. Chem. Soc. 2022, 144 8439. |
| [93] | Ovitt, T. M.; Coates, G. W. J. Am. Chem. Soc. 2002, 124 1316. |
| [94] | Appiah, W. O.; DeGreeff, A. D.; Razidlo, G. L.; Spessard, S. J.; Pink, M.; Young, V. G.; Hofmeister, G. E. Inorg. Chem. 2002, 41 3656. |
| [95] | Chow, H. S.; Constable, E. C.; Housecroft, C. E.; Neuburger, M.; Schaffner, S. Dalton Trans. 2006, 23 2881. |
| [96] | Jalee, K.; Bongki, S.; Hyunjeong, K.; Junhyung, L.; Joongoo, K.; Sachiko, Y.; Takashi, O.; Hideki, M.; Tomohiro, O.; Jaeheung, C. Inorg. Chem. 2015, 54 6176. |
| [97] | Che, C.-M.; Li, Z.-Y.; Wong, K.-Y.; Poon, C.-K.; Mak, T. C. W.; Peng, S.-M. Polyhedron 1994, 13 771. |
/
| 〈 |
|
〉 |