

Radiosynthesis of β-Fluoroamines with 18F
Received date: 2024-08-06
Online published: 2024-11-06
Supported by
National Natural Science Foundation of China(22078161); National Natural Science Foundation of China(22108124)
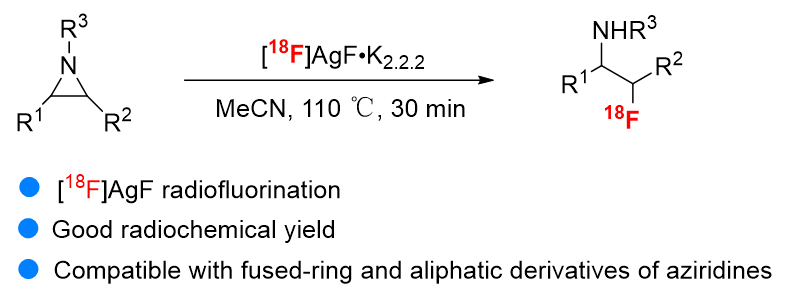
β-Fluoroamines represent a crucial motif in biological and pharmaceutical sciences, largely due to the introduction of fluorine, which enhances the binding affinity, metabolic stability, and bioavailability of target molecules. This makes them highly valuable in the realm of drug discovery. Among the various radionuclides utilized in nuclear medicine imaging, fluorine-18 (¹⁸F) is the most prevalent. Its favorable physicochemical properties, including a half-life of 109.8 min, facilitate optimal target-to-background ratios in imaging processes. Additionally, ¹⁸F is advantageous for multi-step synthesis and transportation, and its decay produces oxygen-18 (¹⁸O) atoms, which are harmless to humans. Given this context, our research aims to synthesize β-fluoroamine positron emission computed tomography (PET/CT) imaging agents with significant potential applications. Developing an effective radiolabeling methodology will greatly advance the in vivo visualization and evaluation of target molecules. In this study, we employed [¹⁸F]AgF in the hydro fluorination of aziridines to achieve the radiosynthesis of [¹⁸F]β-fluoroamines. Preliminary optimizations revealed that additional silver salts and acids were unnecessary, and the labeling process does not require azeotropic drying. This method exhibits good compatibility with light, water, and oxygen, successfully circumventing the complex radioactive dehydration processes while also facilitating automatic labeling. Moreover, the reaction delivered excellent radiochemical conversion (RCC) and moderate radiochemical yield (RCY) in optimized conditions. Radioactive high performance liquid chromatography (Radio-HPLC) spectrum shows that the radiochemical conversion rate of labeled products is more than 80%. Further analysis of the collected radioactive products shows that most of the reactions have a radiation yield of more than 30%. For the key molecule 2a, we conducted a calculation of the specific activity. The higher the specific activity, the greater its potential for biological applications. After three experiments, the specific activity of 2a was determined to be 15.6±2.9 GBq/μmol. Also, the reactions have good compatibility with fused-ring and aliphatic derivatives of aziridines with various function groups. Overall, we have developed a highly efficient and easy-handling radiolabeling method for the radiosynthesis of β-fluoroamines. This solution provides greater flexibility when designing related radiopharmaceuticals.
Zhengxu Yin , Guoqiang Xu , Tengxiang Chen , Junbin Han . Radiosynthesis of β-Fluoroamines with 18F[J]. Acta Chimica Sinica, 2024 , 82(11) : 1120 -1123 . DOI: 10.6023/A24080235
| [1] | Gawne, P. J.; Man, F.; Blower, P. J.; Rosales, T. M. R. Chem. Rev. 2022, 122, 10266. |
| [2] | Schwenck, J.; Sonanini, D.; Cotton, J. M.; Rammensee, H.-G.; la Fougère, C.; Zender, L.; Pichler, B. J. Nat. Rev. Cancer. 2023, 23, 474. |
| [3] | McCluskey, S. P.; Plisson, C.; Rabiner, E. A.; Howes, O. Eur. J. Nucl. Med. Mol. Imaging. 2020, 47, 451. |
| [4] | Deng, Y.-J.; Zhu, H.; Yang, Z.; Peng, Z.-P.; Jia, J.-H. JNRC 2020, 33, 250 (in Chinese). |
| [4] | (邓虞娇, 朱华, 杨志, 彭志平, 贾建华, 核化学与放射化学, 2020, 33, 250.) |
| [5] | Singh, S. B.; Ng, S. J.; Lau, H. C.; Khanal, K.; Bhattarai, S.; Paudyal, P.; Shrestha, B. B.; Naseer, R.; Sandhu, S.; Gokhale, S.; Raynor, W. Y. Cardiol. Ther. 2023, 12, 85. |
| [6] | John, P. S. J. Nucl. Med. 2023, 64, 12. |
| [7] | Rong, J.; Haider, A.; Jeppesen, T. E.; Josephson, L.; Liang, S. H. Nat. Commun. 2023, 14, 3257. |
| [8] | Inoue, M.; Sumii, Y.; Shibata, N. ACS Omega 2020, 5, 10633. |
| [9] | Wang, C.-Q.; Feng, C. Acta Chim. Sinica 2024, 82, 160 (in Chinese). |
| [9] | (王成强, 冯超, 化学学报, 2024, 82, 160.) |
| [10] | Wang, L.-B.; Liu, M.-H.; Zu, Y.-M.; Yao, H.; Wu, C.; Zhang, R.-X.; Ma, W.-N.; Lu, H.-G.; Xi, S.; Hua, L.; Wang, G.-L.; Tang, Y.-F. Eur. J. Med. Chem. 2022, 236, 11426. |
| [11] | Asati, V.; Mahapatra, D. K.; Bharti, S. K. Eur. J. Med. Chem. 2019, 172, 95. |
| [12] | Patel, H.; Pawara, R.; Ansari, A.; Surana, S. Eur. J. Med. Chem. 2017, 142, 32. |
| [13] | Wu, T.; Yin, G.; Liu, G.-S. J. Am. Chem. Soc. 2009, 131, 16354. |
| [14] | Li, Y.; Bao, J.; Zhang, Y.; Peng, X.; Yu, W.; Wang, T.; Yang, D.; Liu, Q.; Zhang, Q.; Fu, J.-K. Chem 2022, 8, 1147. |
| [15] | Dank, C.; Ielo, L. Org. Biomol. Chem. 2023, 21, 4553. |
| [16] | Fan, R.-H.; Zhou, Y.-G.; Zhang, W.-X.; Hou, X.-L.; Dai, L.-X. J. Org. Chem. 2004, 69, 335. |
| [17] | Kalow, J. A.; Schmitt, D. E.; Doyle, A. G. J. Org. Chem. 2012, 77, 4177. |
| [18] | Zhu, L.; Xiong, J.; An, J.; Chen, N.; Xue, J.; Jiang, X.-X. Org. Biomol. Chem. 2019, 17, 3797. |
| [19] | Okoromoba, O. E.; Li, Z.; Robertson, N.; Mashuta, M. S.; Couto, U. R.; Tormena, C. F.; Xu, B.; Hammond, G. B. Chem. Commun. 2016, 52, 13353. |
| [20] | Roehn, U.; Becaud, J.; Mu, L.; Srinivasan, A.; Stellfeld, T.; Fitzner, A.; Graham, K.; Dinkelborg, L.; Schubiger, A. P.; Ametamey, S. M. J. Fluorine Chem. 2009, 130, 902. |
| [21] | Schjoeth-Eskesen, C.; Hansen, P. R.; Kjaer, A.; Gillings, N. ChemistryOpen 2015, 4, 65. |
| [22] | Lee, S. J.; Brooks, A. F.; Ichiishi, N.; Makaravage, K. J.; Mossine, A. V.; Sanford, M. S.; Scott, P. J. H. Chem. Commun. 2019, 55, 2976. |
| [23] | Thompson, S.; Lee, S. J.; Jackson, I. M.; Ichiishi, N.; Brooks, A. F.; Sanford, M. S.; Scott, P. J. H. Synthesis 2019, 51, 4401. |
| [24] | Zhao, Q.; Isenegger Patrick, G.; Wilson Thomas, C.; Sap Jeroen, B. I.; Guibbal, F.; Lu, L.; Gouverneur, V.; Shen, Q.-L. CCS Chem. 2020, 3, 1921. |
| [25] | Gao, X.-Y.; Gong, K.-H.; Wang, M.-W.; Xu, B.; Han, J.-B. Org. Lett. 2022, 24, 6438. |
| [26] | Gong, K.-H.; Yin, Z.-X.; Song, P.-F.; Xu, B.; Han, J.-B. Synlett 2024, 35, 1569. |
/
| 〈 |
|
〉 |