

Research Progress on Copper(I)-Catalyzed Asymmetric Allylation of Ketones or Ketimines
Received date: 2024-10-11
Online published: 2024-11-26
Supported by
National Natural Science Foundation of China(22271302); Natural Science Foundation of Shanxi Province(202303021211189)
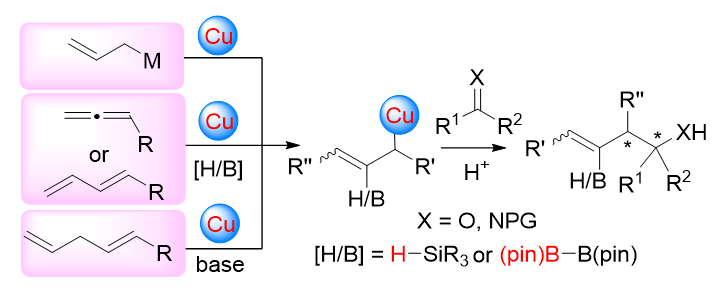
Chiral tertiary homoallylic alcohol and chiral α,α-disubstituted homoallylic amine scaffolds are ubiquitous in numerous bioactive natural products and pharmaceutically relevant molecules. Thus, asymmetric synthesis of these compounds has attracted an increasing attention from synthetic chemists. Compared to traditional methods, transition-metal-catalyzed asymmetric allylation of ketones or ketimines serves as a powerful methodology for constructing these compounds due to its excellent atom- and step-economy. Recent progress on copper(I)-catalyzed asymmetric allylation of ketones or ketimines is summarized. Based on the strategies for the generation of allyl-copper(I) species in situ, this review is divided into three sections: reactions through transmetalation, three-component coupling reactions, and proton-transfer reactions. The mechanisms and potential applications of some representative strategies are also included. Finally, the future developments in this field are outlooked.
Hui Li , Liang Yin . Research Progress on Copper(I)-Catalyzed Asymmetric Allylation of Ketones or Ketimines[J]. Acta Chimica Sinica, 2024 , 82(12) : 1274 -1288 . DOI: 10.6023/A24100300
| [1] | (a) Mizui, Y.; Sakai, T.; Iwata, M.; Uenaka, T.; Okamoto, K.; Shimizu, H.; Yamori, T.; Yoshimatsu, K.; Asada, M. J. Antibiot. 2004, 57, 188. |
| [1] | (b) Paterson, I.; Dalby, S. M.; Roberts, J. C.; Naylor, G. J.; Guzmán, E. A.; Isbrucker, R.; Pitts, T. P.; Linley, P.; Divlianska, D.; Reed, J. K.; Wright, A. E. Angew. Chem., Int. Ed. 2011, 50, 3219. |
| [1] | (c) Friestad, G. K.; Mathies, A. K. Tetrahedron 2007, 63, 2541. |
| [2] | (a) Pu, L.; Yu, H.-B. Chem. Rev. 2001, 101, 757. |
| [2] | (b) Shibasaki, M.; Kanai, M. Chem. Rev. 2008, 108, 2853. |
| [3] | (a) Read, J. A.; Woerpel, K. A. J. Org. Chem. 2017, 82, 2300. |
| [3] | (b) Bartolo, N. D.; Woerpel, K. A. J. Org. Chem. 2018, 83, 10197. |
| [4] | Cervera-Padrell, A. E.; Nielsen, J. P.; Pedersen, M. J.; Christensen, K. M.; Mortensen, A. R.; Skovby, T.; Dam-Johansen, K.; Kiil, S.; Gernaey, K. V. Org. Process Res. Dev. 2012, 16, 901. |
| [5] | Yamasaki, S.; Fujii, K.; Wada, R.; Kanai, M.; Shibasaki, M. J. Am. Chem. Soc. 2002, 124, 6536. |
| [6] | Wada, R.; Oisaki, K.; Kanai, M.; Shibasaki, M. J. Am. Chem. Soc. 2004, 126, 8910. |
| [7] | Kanai, M.; Wada, P.; Shibuguchi, T.; Shibasaki, M. Pure Appl. Chem. 2008, 80, 1055. |
| [8] | Shi, S.-L.; Xu, L.-W.; Oisaki, K.; Kanai, M.; Shibasaki, M. J. Am. Chem. Soc. 2010, 132, 6638. |
| [9] | Motoki, R.; Tomita, D.; Kanai, M.; Shibasaki, M. Tetrahedron Lett. 2006, 47, 8083. |
| [10] | Iwamoto, H.; Hayashi, Y.; Ozawa, Y.; Ito, H. ACS Catal. 2020, 10, 2471. |
| [11] | Zanghi, J. M.; Meek, S. J. Angew. Chem., Int. Ed. 2020, 59, 8451. |
| [12] | Sun, B.; Ruan, L.-X.; Zhao, R.; Zhang, J.; Niu, R.; Luo, Q.; Zhang, Y.; Gao, L.; Shi, S.-L. Nat. Synth. 2024, 3, 1091. |
| [13] | Wada, R.; Shibuguchi, T.; Makino, S.; Oisaki, K.; Kanai, M.; Shibasaki, M. J. Am. Chem. Soc. 2006, 128, 7687. |
| [14] | Tsai, E. Y.; Liu, R. Y.; Yang, Y.; Buchwald, S. L. J. Am. Chem. Soc. 2018, 140, 2007. |
| [15] | Liu, R. Y.; Zhou, Y.; Yang, Y.; Buchwald, S. L. J. Am. Chem. Soc. 2019, 141, 2251. |
| [16] | Xu, M.; Lu, Q.; Gong, B.; Ti, W.; Lin, A.; Yao, H.; Gao, S. Angew. Chem., Int. Ed. 2023, 62, e202311540. |
| [17] | (a) Gupta, P.; Mahajan, N. New J. Chem. 2018, 42, 12296. |
| [17] | (b) Mushtaq, S.; Abbasi, B. H.; Uzair, B.; Abbasi, R. EXCLI Journal 2018, 17, 420. |
| [18] | Klake, R. K.; Edwards, M. D.; Sieber, J. D. Org. Lett. 2021, 23, 6444. |
| [19] | Collins, S.; Sieber, J. D. Org. Lett. 2023, 25, 1425. |
| [20] | Min, L.; Han, J.-C.; Zhang, W.; Gu, C.-C.; Zou, Y.-P.; Li, C.-C. Chem. Rev. 2023, 123, 4934. |
| [21] | Zhou, M.; Lin, Y.; Chen, X.-X.; Xu, G.; Chung, L. W.; Tang, W. Angew. Chem., Int. Ed. 2023, 62, e202300334. |
| [22] | Jiang, N.; Liu, P.-Z.; Pan, Z.-Z.; Wang, S.-Q.; Peng, Q.; Yin, L. Angew. Chem., Int. Ed. 2024, 63, e202402195. |
| [23] | Yang, Y.; Perry, I. B.; Lu, G.; Liu, P.; Buchwald, S. L. Science 2016, 353, 144. |
| [24] | Li, C.; Liu, R. Y.; Jesikiewicz, L. T.; Yang, Y.; Liu, P.; Buchwald, S. L. J. Am. Chem. Soc. 2019, 141, 5062. |
| [25] | Fu, B.; Yuan, X.; Li, Y.; Wang, Y.; Zhang, Q.; Xiong, T.; Zhang, Q. Org. Lett. 2019, 21, 3576. |
| [26] | (a) McManus, H. A.; Fleming, M. J.; Lautens, M. Angew. Chem., Int. Ed. 2007, 46, 433. |
| [26] | (b) Perrone, R.; Berardi, F.; Colabufo, N. A.; Leopoldo, M.; Tortorella, V.; Fiorentini, F.; Olgiati, V.; Ghiglieri, A.; Govoni, S. J. Med. Chem. 1995, 38, 942. |
| [27] | Acharyya, R. K.; Kim, S.; Park, Y.; Han, J. T.; Yun, J. Org. Lett. 2020, 22, 7897. |
| [28] | Zhu, J.; Rahim, F.; Zhou, P.; Zhang, A.; Malcolmson, S. J. J. Am. Chem. Soc. 2024, 146, 20270. |
| [29] | (a) Tewes, B.; Frehland, B.; Schepmann, D.; Robaa, D.; Uengwetwanit, T.; Gaube, F.; Winckler, T.; Sippl, W.; Wu?nsch, B. J. Med. Chem. 2015, 58, 6293. |
| [29] | (b) Novoa, A.; Van Dorpe, S.; Wynendaele, E.; Spetea, M.; Bracke, N.; Stalmans, S.; Betti, C.; Chung, N. N.; Lemieux, C.; Zuegg, J.; Cooper, M. A.; Tourwé, D.; De Spiegeleer, B.; Schiller, P. W.; Ballet, S. J. Med. Chem. 2012, 55, 9549. |
| [30] | Li, D.; Park, Y.; Yoon, W.; Yun, H.; Yun, J. Org. Lett. 2019, 21, 9699. |
| [31] | Deng, X.-H.; Jiang, J.-X.; Jiang, Q.; Yang, T.; Chen, B.; He, L.; Chu, W.-D.; He, C.-Y.; Liu, Q.-Z. Org. Lett. 2022, 24, 4586. |
| [32] | Meng, F.; Jang, H.; Jung, B.; Hoveyda, A. H. Angew. Chem., Int. Ed. 2013, 52, 5046. |
| [33] | Zhao, Y.-S.; Tang, X.-Q.; Tao, J.-C.; Tian, P.; Lin, G.-Q. Org. Biomol. Chem. 2016, 14, 4400. |
| [34] | (a) Yeung, K.; Talbot, F. J. T.; Howell, G. P.; Pulis, A. P.; Procter, D. J. ACS Catal. 2019, 9, 1655. |
| [34] | (b) Deng, H.; Dong, Y.; Yu, S.; Yang, F.; Han, S.; Wu, J.; Liang, B.; Guo, H.; Zhang, C. Org. Lett. 2021, 23, 4431. |
| [34] | (c) Ashraf, M. A.; Tambe, S. D.; Cho, E. J. Bull. Korean Chem. Soc. 2021, 42, 683. |
| [35] | Jang, H.; Romiti, F.; Torker, S.; Hoveyda, A. H. Nat. Chem. 2017, 9, 1269. |
| [36] | Zhao, C.-Y.; Zheng, H.; Ji, D.-W.; Min, X.-T.; Hu, Y.-C.; Chen, Q.-A. Cell Rep. Phys. Sci. 2020, 1, 100067. |
| [37] | Liu, X.; Shi, S. Chin. J. Org. Chem. 2024, 44, 1884. |
| [38] | Feng, J.-J.; Xu, Y.; Oestreich, M. Chem. Sci. 2019, 10, 9679. |
| [39] | Yoon, W. S.; Han, J. T.; Yun, J. Adv. Synth. Catal. 2021, 363, 4953. |
| [40] | Li, D.; Park, Y.; Yun, J. Org. Lett. 2018, 20, 7526. |
| [41] | Yazaki, R.; Kumagai, N.; Shibasaki, M. J. Am. Chem. Soc. 2010, 132, 5522. |
| [42] | Wei, X.-F.; Xie, X.-W.; Shimizu, Y.; Kanai, M. J. Am. Chem. Soc. 2017, 139, 4647. |
| [43] | Zhong, F.; Pan, Z.-Z.; Zhou, S.-W.; Zhang, H.-J.; Yin, L. J. Am. Chem. Soc. 2021, 143, 4556. |
| [44] | Liu, J.; Su, B.; Chen, M. Org. Lett. 2021, 23, 6035. |
| [45] | Pan, Z.-Z.; Li, J.-H.; Tian, H.; Yin, L. Angew. Chem., Int. Ed. 2024, 63, e202315293. |
| [46] | Pan, Z.-Z.; Pan, D.; Li, J.-H.; Xue, X.-S.; Yin, L. J. Am. Chem. Soc. 2023, 145, 1749. |
| [47] | (a) Fu, Z.; Xu, J.; Zhu, T.; Leong, W. W. Y.; Chi, Y. R. Nat. Chem. 2013, 5, 835. |
| [47] | (b) Xie, Y.; Yu, C.; Li, T.; Tu, S.; Yao, C. Chem. Eur. J. 2015, 21, 5355. |
| [47] | (c) Ma, J.; Rosales, A. R.; Huang, X.; Harms, K.; Riedel, R.; Wiest, O.; Meggers, E. J. Am. Chem. Soc. 2017, 139, 17245. |
/
| 〈 |
|
〉 |