

Ring-opening Polymerization of rac-Lactide Catalyzed by Zinc Chloride Complexes Supported by Aminophenolate Ligands Bearing a Benzoxazolyl Group
Received date: 2024-12-23
Online published: 2025-01-07
Supported by
National Natural Science Foundation of China(21871082)
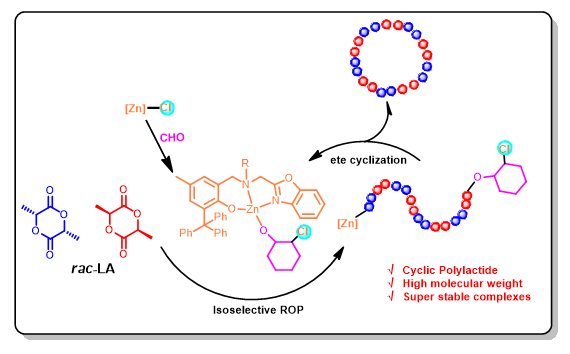
Four zinc chloride complexes supported by aminophenolate ligands bearing a benzoxazolyl group were synthesized via the reactions of the corresponding sodium salts of the proligands HL1-4 with one equiv. of zinc dichloride at room temperature respectively, and their structures were well-characterized by 1H NMR, 13C NMR and elemental analysis (EA) methods. The molecular structure of complex 1 was further determined by X-ray single crystal diffraction analysis, where the phenoxy oxygen atom, the skeleton N atom, and the benzoxazolyl N atom of the ligand all coordinate with the metal center, forming a disordered tetrahedral coordination geometry around the metal center together with the chloride ligand. This series of zinc chloride complexes showed high stability, and could be applied to catalyze the ring-opening polymerization (ROP) of industrial grade racemic lactide (rac-LA) with good activities. The substituent on the skeleton N atom exhibited a significant impact on the catalytic performance of the complex. Complex 1 with a linear n-butyl group on the skeleton N atom displayed the highest catalytic activity. At 80 ℃, 50000 equiv. of industrial grade rac-LA could be polymerized by complex 1 using cyclohexane oxide (CHO) as a solvent, and a high turnover frequency (TOF) value of 2716 h-1 was reached, resulting in a cyclic polymer with a molecular weight of Mn=2.03×104 g/mol. Complex 3 with a cyclohexyl group on the skeleton N atom showed the highest isotactic selectivity among these zinc chloride complexes toward the ROP of rac-LA, at 80 ℃, Pm=0.67, which could be increased to Pm=0.70 when the polymerization was carried out at 25 ℃. By adopting a high monomer concentration of 14 mol/L in the polymerization, cyclic polylactide with a molecular weight of Mn=3.10×104 g/mol was achieved using complex 1. Based on the matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectroscopic analysis of oligomers, it was found that in the absence of benzyl alcohol, the polymer formed was mainly cyclic, containing small amount of linear chains end-capped with -OH/-COOH, -OH/-OC6H10Cl groups etc.; while in the presence of benzyl alcohol, the resultant polymer was found to be a mixture of linear ones end-capped with -OH/-OBn and cyclic ones, both in a reasonable amount. It is thus suggested that, in the absence of external alcohol a coordination-insertion polymerization mechanism is involved, which integrates with end-to-end and non-selective cyclization to produce mainly cyclic polymers, wherein CHO acts as activator to zinc chloride complexes by forming zinc alkoxide species via the nucleophilic attack of chlorine to the coordinated CHO; when benzyl alcohol was used, it acts as a chain transfer reagent with the active propaga- tion chain end-capped with -OH/-OC6H10Cl to generate zinc benzyloxide species, which initiates the ROP of rac-LA to form linear polymers end-capped with -OH/-OBn groups, meanwhile the cyclization of the active propagation chain end-capped with -OH/-OC6H10Cl still takes place in this system.
Yuna Wang , Chao Wang , Haiyan Ma . Ring-opening Polymerization of rac-Lactide Catalyzed by Zinc Chloride Complexes Supported by Aminophenolate Ligands Bearing a Benzoxazolyl Group[J]. Acta Chimica Sinica, 2025 , 83(1) : 25 -35 . DOI: 10.6023/A24090275
| [1] | Zhu, Y.; Romain, C.; Williams, C. K. Nature 2016, 540, 354. |
| [2] | Keram, M.; Ma, H. Acta Chim. Sinica 2018, 76, 121 (in Chinese) |
| [2] | ( 布美热木?克里木, 马海燕, 化学学报, 2018, 76, 121.) |
| [3] | Pappalardo, D.; Mathisen, T.; Finne-Wistrand, A. Biomacromolecules 2019, 20, 1465. |
| [4] | Mehta, R.; Kumar, V.; Bhumia, H.; Upadhyay, S. N. J. Macromol. Sci. Part C: Polym. Rev. 2005, 45, 325. |
| [5] | Drumright, R. E.; Gruber, P. R.; Henton, D. E. Adv. Mater. 2000, 12, 1841. |
| [6] | Ungpittagul, T.; Wongmahasirikun, P.; Phomphrai, K. Dalton Trans. 2020, 49, 8460. |
| [7] | Haque, F. M.; Grayson, S. M. Nat. Chem. 2020, 12, 433. |
| [8] | Pangilinan, K.; Advincula, R. Polym. Int. 2014, 63, 803. |
| [9] | Chang, Y. A.; Waymouth, R. M. J. Polym. Sci., Part A: Polym. Chem. 2017, 55, 2892. |
| [10] | Laurent, B. A.; Grayson, S. M. Chem. Soc. Rev. 2009, 38, 2202. |
| [11] | Hu, C.; Louisy, E.; Fontaine, G.; Bonnet, F. J. Polym. Sci., Part A: Polym. Chem. 2017, 55, 3175. |
| [12] | Piedra-Arroni, E.; Ladaviere, C.; Amgoune, A.; Bourissou, D. J. Am. Chem. Soc. 2013, 135, 13306. |
| [13] | Shaik, M.; Peterson, J.; Du, G. Macromolecules 2019, 52, 157. |
| [14] | Liu, J. Y.; Liu, S.; Suo, H. Y.; Qin, Y. S. Acta Polym. Sin. 2024, 55, 770 (in Chinese). |
| [14] | ( 刘娇玉, 刘爽, 索泓一, 秦玉升, 高分子学报, 2024, 55, 770.) |
| [15] | Culkin, D. A.; Jeong, W.; Csihony, S.; Gomez, E. D.; Balsara, N. P.; Hedrick, J. L.; Waymouth, R. M. Angew. Chem., Int. Ed. 2007, 46, 2627. |
| [16] | Shin, E. J.; Brown, H. A.; Gonzalez, S.; Jeong, W.; Hedrick, J. L.; Waymouth, R. M. Angew. Chem., Int. Ed. 2011, 50, 6388. |
| [17] | Stukenbroeker, T. S.; Solis-Ibarra, D.; Waymouth, R. M. Macromolecules 2014, 47, 8224. |
| [18] | Chang, Y. A.; Rudenko, A. E.; Waymouth, R. M. ACS Macro Lett. 2016, 5, 1162. |
| [19] | Dunn, A. L.; Landis, C. R. Macromolecules 2017, 50, 2267. |
| [20] | Weil, J.; Mathers, R. T.; Getzler, Y. D. Y. L. Macromolecules 2012, 45, 1118. |
| [21] | Castro-Osma, J. A.; Alonso-Moreno, C.; García-Martinez, J. C.; Fernández-Baeza, J.; Sánchez-Barba, L. F.; Lara-Sánchez, A.; Otero, A. Macromolecules 2013, 46, 6388. |
| [22] | Weidner, S. M.; Kricheldorf, H. R. Macromol. Chem. Phys. 2017, 218, 1600331. |
| [23] | Anker, M.; Balasanthiran, C.; Balasanthiran, V.; Chisholm, M. H.; Jayaraj, S.; Mathieu, K.; Piromjitpong, P.; Praban, S.; Raya, B.; Simonsick, W. J. Dalton Trans. 2017, 46, 5938. |
| [24] | Praban, S.; Piromjitpong, P.; Balasanthiran, V.; Jayaraj, S.; Chisholm, M. H.; Tantirungrotechai, J.; Phomphrai, K. Dalton Trans. 2019, 48, 3223. |
| [25] | Praban, S.; Yimthachote, S.; Kiriratnikom, J.; Chotchatchawankul, S.; Tantirungrotechai, J.; Phomphrai, K. J. Polym. Sci., Part A: Polym. Chem. 2019, 57, 2104. |
| [26] | Kricheldorf, H. R.; Weidner, S. M.; Meyer, A. Polym. Chem. 2020, 11, 2182. |
| [27] | Kerr, R. W. F.; Ewing, P. M. D. A.; Raman, S. K.; Smith, A. D.; Williams, C. K.; Arnold, P. L. ACS Catal. 2021, 11, 1563. |
| [28] | Piromjitpong, P.; Ratanapanee, P.; Thumrongpatanaraks, W.; Kongsaeree, P.; Phomphrai, K. Dalton. Trans. 2012, 41, 12704. |
| [29] | Chen, C.; Cui, Y.; Mao, X.; Pan, X.; Wu, J. Macromolecules 2017, 50, 83. |
| [30] | Si, G.; Zhang, S.; Pang, W.; Wang, F.; Tan, C. Polymer 2018, 154, 148. |
| [31] | Impemba, S.; Della Monica, F.; Grassi, A.; Capacchione, C.; Milione, S. ChemSusChem 2020, 13, 141. |
| [32] | Goonesinghe, C.; Jung, H.-J.; Roshandel, H.; Diaz, C.; Baalbaki, H. A.; Nyamayaro, K.; Ezhova, M.; Hosseini, K.; Mehrkhodavandi, P. ACS Catal. 2022, 12, 7677. |
| [33] | Hu, J.; Kan, C.; Ma, H. Inorg. Chem. 2018, 57, 11240. |
| [34] | Gong, Y.; Ma, H. Chem. Commun. 2019, 55, 10112. |
| [35] | Hu, J.; Kan, C.; Wang, H.; Ma, H. Macromolecules 2018, 51, 5304. |
| [36] | Wang, H.; Ma, H. Macromolecules 2024, 57, 6156. |
| [37] | Industrial grade lactides contain trace amounts of protonic impurities such as water and lactic acid. For the vast majority of metal complexes reported in literature, complete decomposition usually occurs when industrial grade lactides are adopted for polymerization directly without purfication. Although zinc chloride complexes show significantly increased tolerance to impurities, they still undergo decomposition to a certain degree, leading to a decrease in catalytic activity. |
| [38] | Larrow, J. F.; Jacobsen, E. N.; Gao, Y.; Hong, Y.; Nie, X.; Zepp, C. M. J. Org. Chem. 1994, 59, 1939. |
/
| 〈 |
|
〉 |